Dipyridamole self-emulsifying medicament administration system and preparation method thereof
A drug delivery system and dipyridamole technology, which are applied in the field of dipyridamole self-emulsifying drug delivery system and its preparation, can solve the problem of no relevant reports on the preparation method, and are suitable for large-scale production and improve bioavailability. , the effect of reducing individual differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Prescription composition:
[0028] Dipyridamole 18g
[0029] Oleic acid 120g
[0030] Labrafac lipophile WL 1349 60g
[0031] Solutol HS 15 260g
[0032] Isopropanol 160g
[0033]
[0034] Made 1000 softgels in total
[0035] Preparation Process:
[0036] The excipients in the prescribed amount are weighed, mixed evenly, then the drug in the prescribed amount is added, stirred to dissolve the drug completely, and made into soft capsules, namely dipyridamole self-emulsifying soft capsules.
[0037] The characteristics of the prepared dipyridamole self-emulsifying drug delivery system:
[0038] Physical and chemical properties:
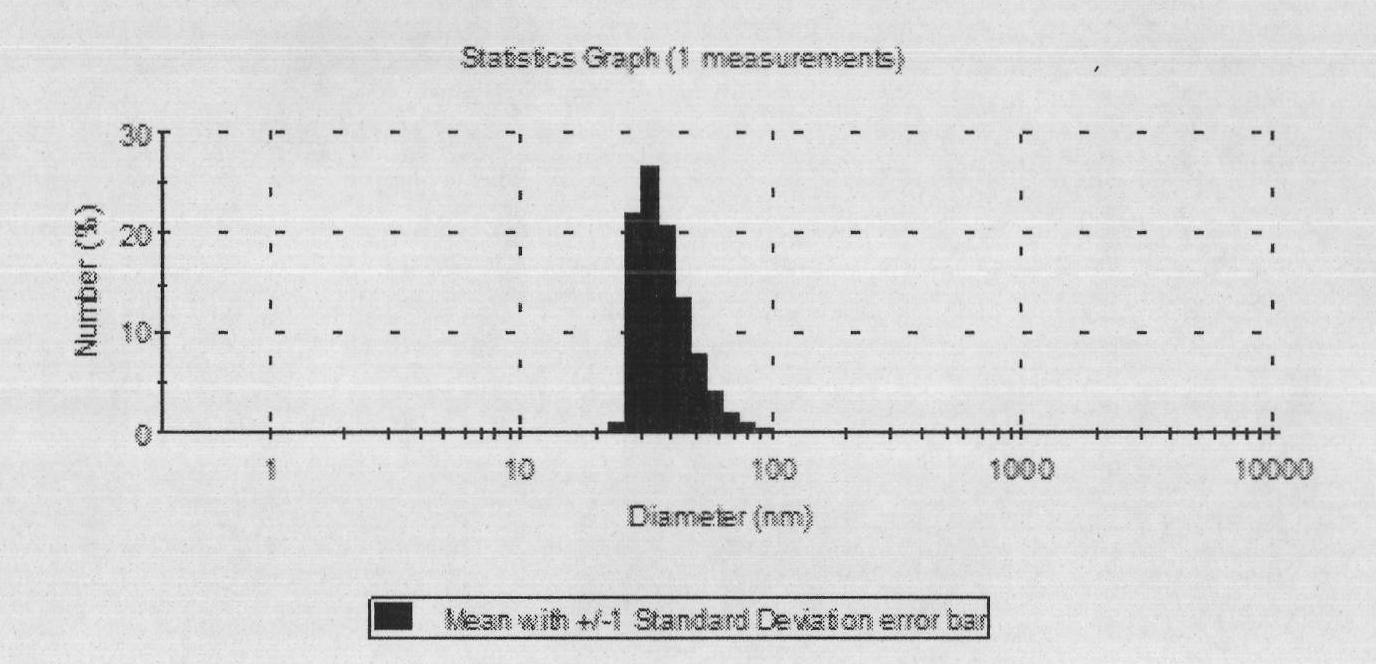
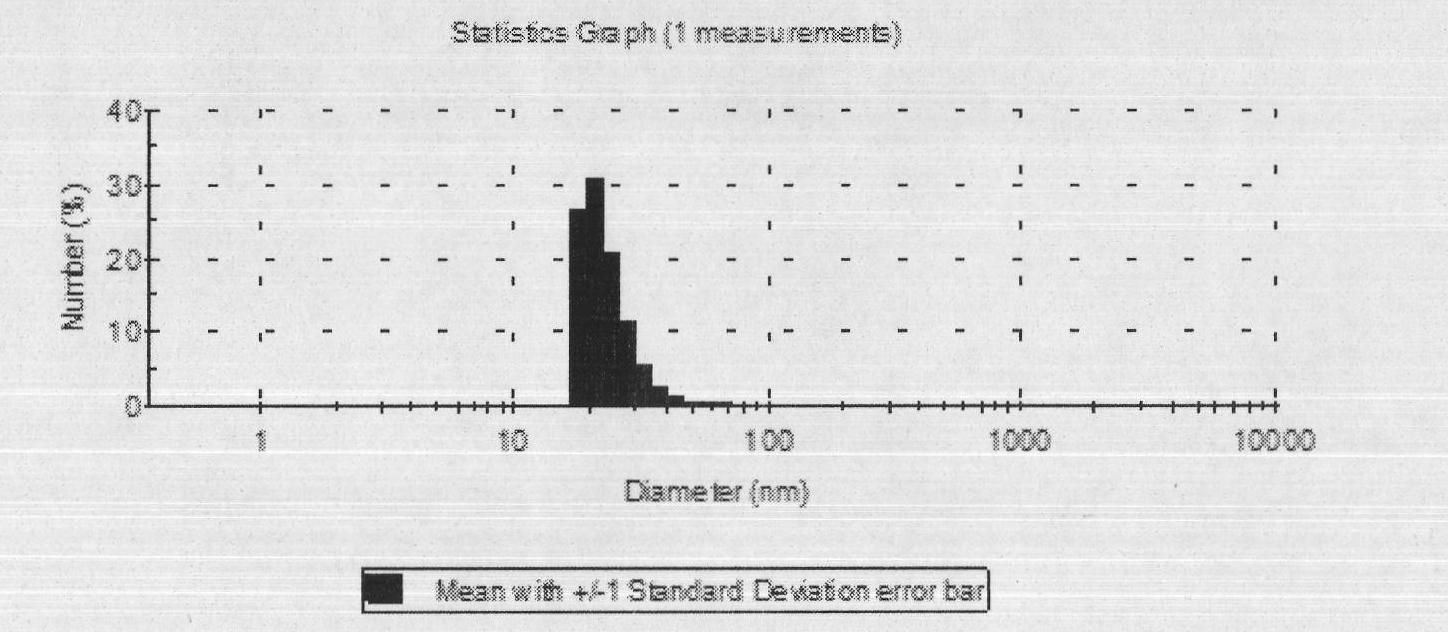
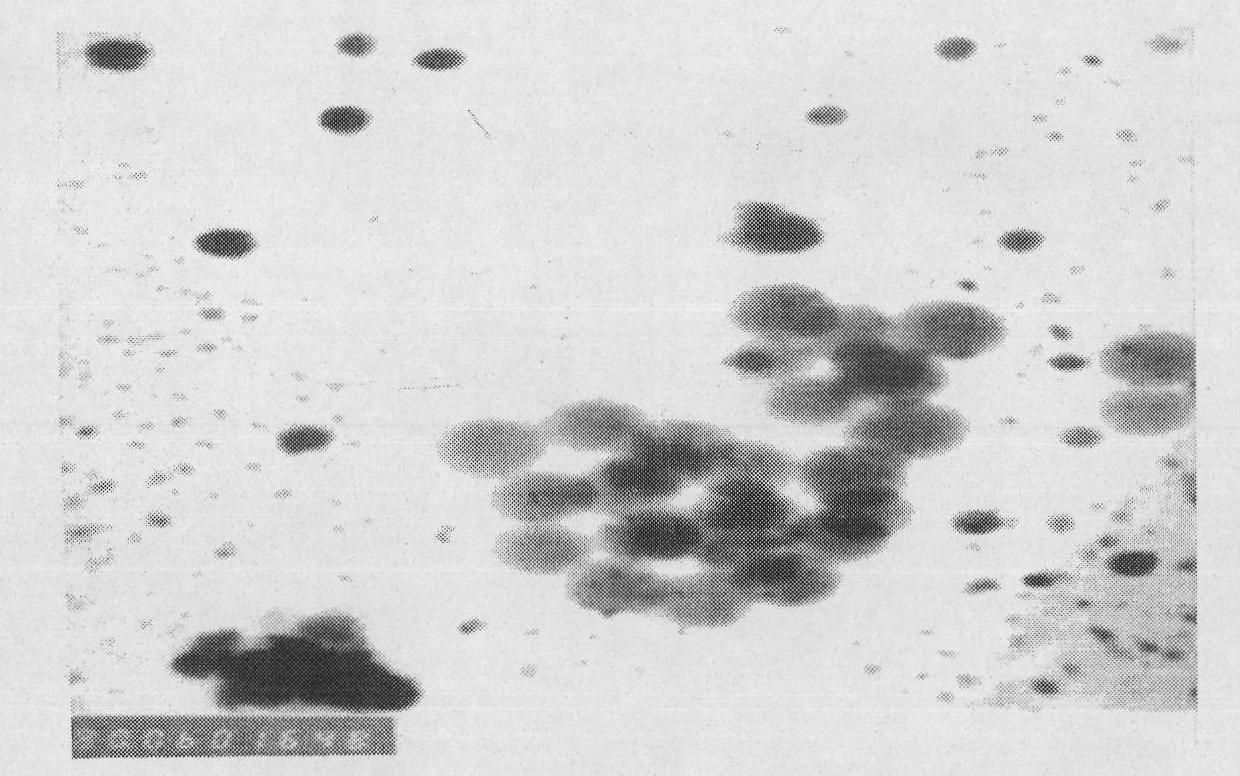
[0039] Particle size determination: Take 50 μl blank self-emulsifying concentrate and dipyridamole self-emulsifying concentrate respectively and add them to a 100ml volumetric flask, dilute to the mark with distilled water, and shake gently to form a clear microemulsion....
Embodiment 2
[0053] Prescription composition:
[0054] Dipyridamole 20g
[0055] Oleic acid 140g
[0056] Labrafac lipophile WL 1349 40g
[0057] Solutol HS 15 240g
[0058] Isopropanol 180g
[0059]
[0060] Made 1000 softgels in total
[0061] Preparation Process:
[0062] The excipients in the prescribed amount are weighed, mixed evenly, then the drug in the prescribed amount is added, stirred to dissolve the drug completely, and made into soft capsules, namely dipyridamole self-emulsifying soft capsules.
Embodiment 3
[0064] Prescription composition:
[0065] Dipyridamole 16g
[0066] Oleic acid 120g
[0067] Labrafac lipophile WL 1349 60g
[0068] Solutol HS 15 260g
[0069] Isopropanol 160g
[0070]
[0071] Made 1000 softgels in total
[0072] Preparation Process:
[0073] The excipients in the prescribed amount are weighed, mixed evenly, then the drug in the prescribed amount is added, stirred to dissolve the drug completely, and made into soft capsules, namely dipyridamole self-emulsifying soft capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com