Preparation method of ginkgo dipyridolum injection with high content of ginkgo terpene lactones
A technology of Damo injection and terpene lactone, which is applied in the field of preparation of Ginkgo Damo injection, can solve the problems affecting the content of terpene lactone, safety risk, and effect on product efficacy, so as to improve the extraction rate and avoid The effect of destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
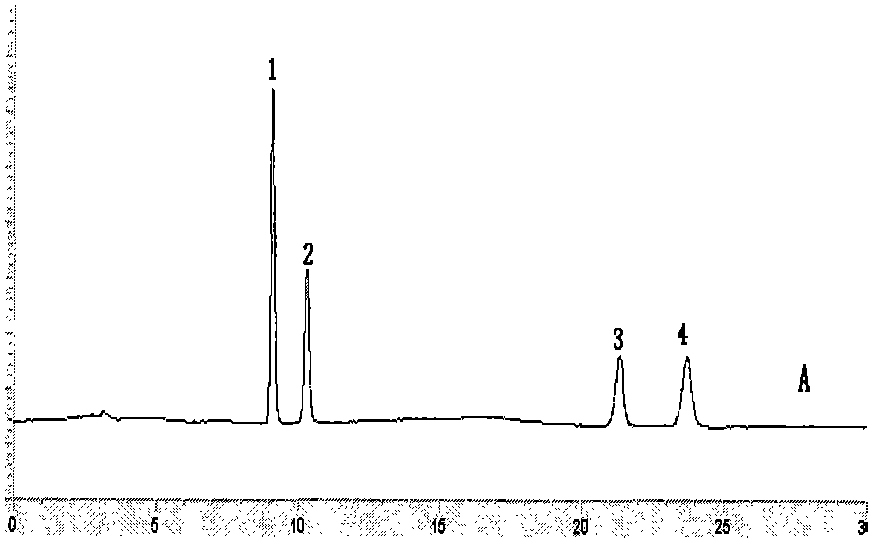
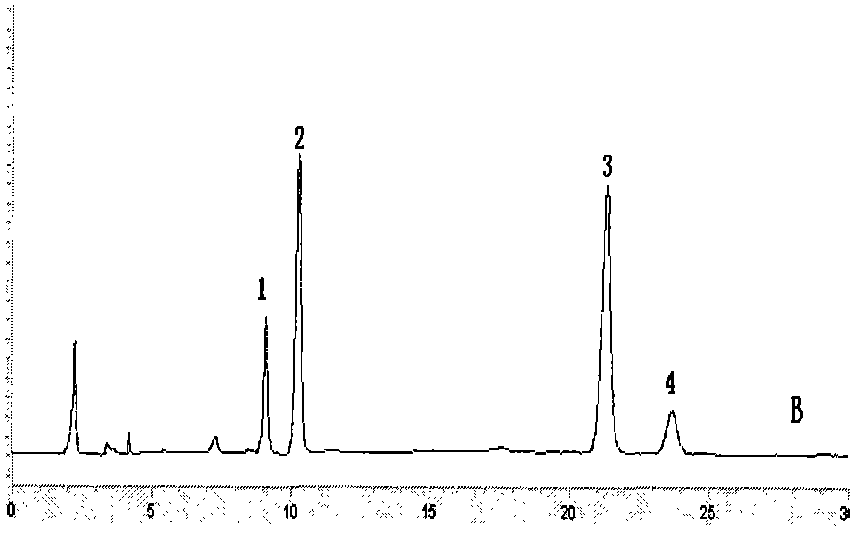
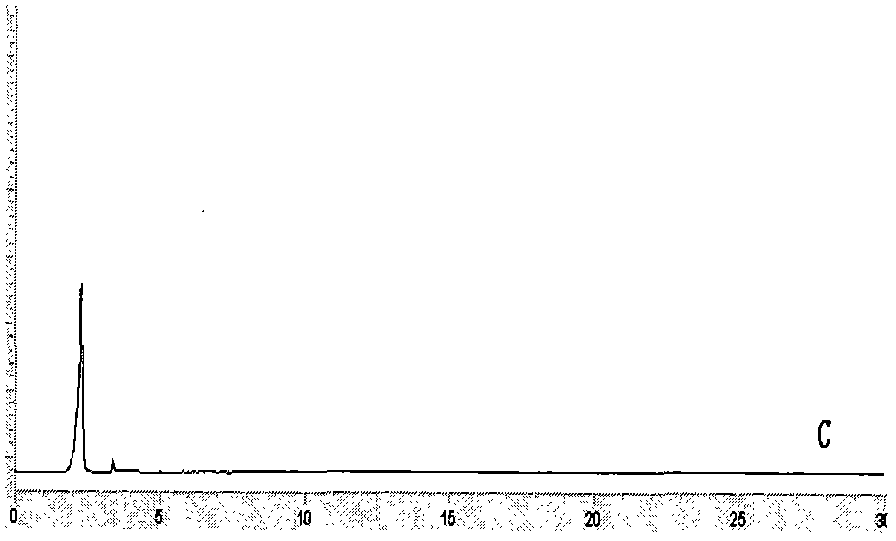
Image
Examples
Embodiment 1
[0031]1. Raw material of Ginkgo biloba: Ginkgo biloba leaves are collected from the artificially planted leaves of Ginkgo biloba. The age of the tree is generally 6-15 years. The water content is controlled below 6%, and it is packaged and stored in a ventilated and dry place. The total content of ginkgolide A, ginkgolide B, ginkgolide C and bilobalide in ginkgo leaves is determined to be 0.361%, and the content of total flavonoids is determined. The content is 0.897%.
[0032] 2. Ginkgo biloba terpenoid lactone extract: put Ginkgo biloba leaves into the extraction device, add 70% ethanol solution with a concentration of 10 times the weight of the medicinal materials, heat and reflux for extraction, extract for 2 hours, and extract twice. The extracts are combined, the solvent is recovered under reduced pressure at low temperature, concentrated and dried, and the organic solvent crude extract of ginkgo leaves is obtained, leaving the extracted ginkgo leaf medicinal residues. ...
Embodiment 2
[0044] 1, Ginkgo biloba raw material: with embodiment 1.
[0045] 2. Ginkgo biloba terpenoid lactone extract: put the ginkgo leaves into the extraction device, add 80% methanol solution according to 7 times the weight of the medicinal materials, heat and reflux for extraction, extract for 3 hours, and extract 3 times in total. The extracts are combined, the solvent is recovered under reduced pressure at low temperature, concentrated and dried, and the organic solvent crude extract of ginkgo leaves is obtained, leaving the extracted ginkgo leaf medicinal residues. The crude extract was added with 4 times the amount of ethyl acetate of the crude extract, extracted and then filtered to obtain the filtrate and the crude extract 2 after extraction with ethyl acetate. Combine the filtrates, reclaim ethyl acetate from the filtrate under reduced pressure, add 90% ethanol solution to fully dissolve, filter, put the filtrate on the treated polyamide chromatographic column, elute with 90...
Embodiment 3
[0056] 1, Ginkgo biloba raw material: with embodiment 1.
[0057] 2. Ginkgo biloba terpenoid lactone extract: put the ginkgo leaves into the extraction device, add 15 times the weight of the medicinal material, add acetone solution with a concentration of 40%, heat and reflux for extraction, extract for 2 hours, and extract twice. The extracts are combined, the solvent is recovered under reduced pressure at low temperature, concentrated and dried, and the organic solvent crude extract of ginkgo leaves is obtained, leaving the extracted ginkgo leaf medicinal residues. Add 3 times the amount of ethyl acetate to the crude extract, extract and filter to obtain the filtrate and the crude extract 2 after extraction with ethyl acetate. Combine the filtrates, recover ethyl acetate from the filtrate under reduced pressure, add 100% ethanol solution to fully dissolve, filter, and put the filtrate on the treated polyamide chromatographic column, elute with the same (specifically what?) s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com