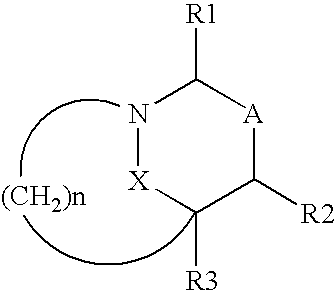
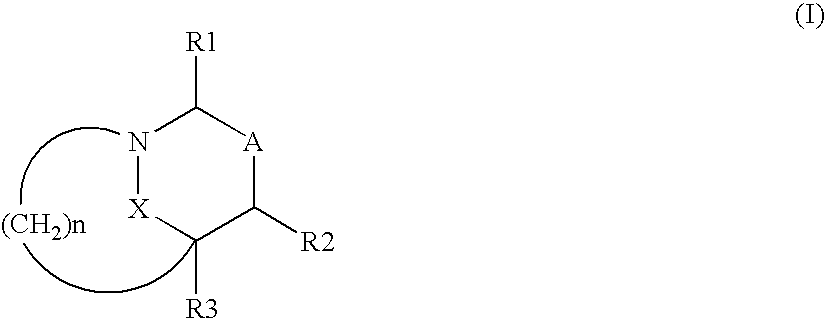
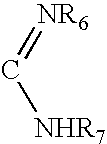Heterocyclic compounds as inhibitors of beta-lactamases
a technology of beta-lactamases and heterocyclic compounds, which is applied in the direction of antibacterial agents, drug compositions, biocides, etc., to achieve the effect of inhibiting the production of -lactamases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
cis diphenylmethyl 7-oxo-6-oxa-1-azabicyclo[3.2.1]octane-4-propanoate
[0144]3.16 g (10.6 mmoles) of the hydrochloride of 3-oxo-1-(phenylmethyl)-4-piperidinepropanoic acid (M=297.7 g) (described in the Japanese Patent Application J54098-772) is mixed with 100 ml of ethanol and the reaction medium is cooled down to 10° C. 1.84 g of NaBH4 is added over 15 minutes, under a stream of nitrogen, whilst maintaining the temperature between 8 and 13° C. The temperature is allowed to rise to ambient temperature and left in contact for 1 hour 30 minutes. Another 380 mg of NaBH4 is added and the reaction medium is left overnight at ambient temperature.
[0145]The solvent is evaporated off under reduced pressure, followed by taking up in 50 ml of water and adjusting the pH from 10 to 2 using concentrated hydrochloric acid. Evaporation is again carried out under reduced pressure. The solid residue (approximately 10.8 g) is washed twice with 100 ml of ethanol then the solvent is evaporated off under r...
example 1a
cis-7-oxo-6-oxa-1-azabicyclo[3.2.1]octane-4-propanoic acid
[0170]176 mg (0.482 mmoles) of the product obtained previously is dissolved in 10 ml of acetone. 90 mg of Pd / C at 10% by weight is added.
[0171]The reaction medium is left to react under a hydrogen atmosphere at normal pressure for 3 hours. Another 25 mg of catalyst is added and the reaction is left to continue for 1 hour 15 minutes.
[0172]The catalyst is filtered then the solvent is evaporated off under reduced pressure in order to obtain 146 mg of product.
[0173]The medium is reacted in 10 ml of acetone with 35 mg of Pd / C at 10% by weight under a hydrogen atmosphere and the reaction is left for 1 hour to complete.
[0174]The catalyst is then separated by filtration and the filtrate is evaporated under reduced pressure. 137 mg of crude product is obtained which is crystallized from a mixture of ethyl ether and petroleum ether. In this way 75 mg of the sought product (M=199 g) is obtained, i.e. a yield of 78%.
[0175]1H NMR
[0176]In ...
example 2
Trans diphenylmethyl 7-oxo-6-oxa-1-azabicyclo[3.2.1]octan-4-acetate
[0180]94 mg (0.259 mmoles) of the compound trans diphenylmethyl 3-hydroxy-4-piperidine-acetate hydrochloride (M=361.87 g) (described in Eur. J. Med. Chem—Chim. Ther—1982-17(6)531-5) and 7 ml of dichloromethane are mixed under an inert atmosphere.
[0181]The reaction medium is cooled down using an ice bath and 19 μl of diphosgene is injected. Agitation is carried out for 25 minutes, then 72 μl of TEA is injected. Agitation is carried out at ambient temperature for 30 minutes and the solvent is evaporated off under reduced pressure, followed by taking up in 7 ml of toluene.
[0182]36 μl of TEA then 31 mg of DMAP are added.
[0183]Heating is carried out for 15 minutes at 100° C., then the reaction medium is left to return to ambient temperature, followed by washing twice with 4 ml of 10% tartaric acid in water, then with 4 ml of water saturated with sodium chloride.
[0184]After drying over magnesium sulphate, filtration is car...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com