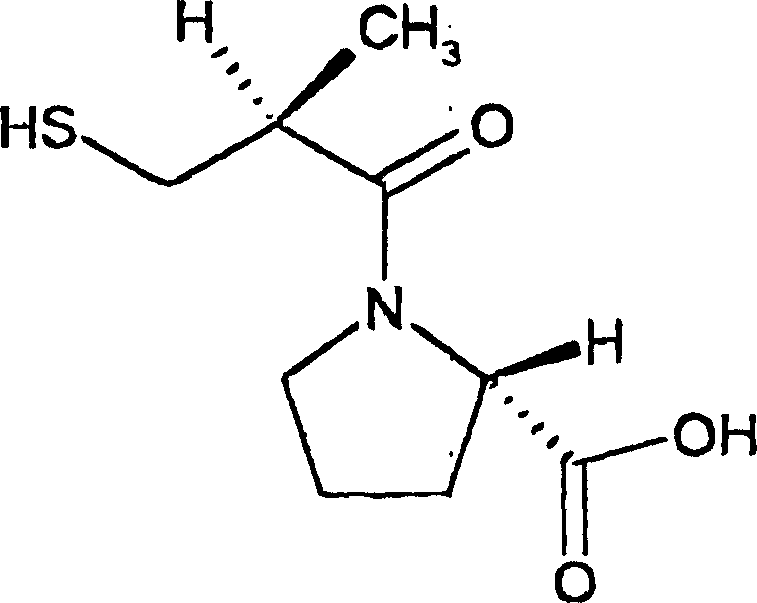
Medicinal composition contg. katopril, and its prepn. method
A composition and drug technology, applied in the pharmaceutical composition containing captopril and its preparation field, can solve the problems of high disulfide content, complicated process, unstable product content, etc., and achieve the effect of stable product content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Stir 6 kg of lactose, 1 kg of starch, 1.5 kg of pregelatinized starch, and 1 kg of hydroxypropyl cellulose, pass through a 16-mesh sieve, and add PVP with a weight percentage concentration of 5% by a method known in the art. It is a 50% ethanol solution, in which, 210 g of povidone K30 (PVP.k30) is wet granulated, dried at 80°C for 4 hours, dried for 2 hours and tumbled, and the granules are granulated through a 16-mesh sieve;
[0033] (2) 2.55 kg of captopril and 2.5 kg of microcrystalline cellulose were stirred evenly, passed through a 16-mesh sieve, dry-pressed for sizing, and the roller pressure of the dry press was set at 5Mpa;
[0034] (3) With the above two kinds of granules, add 0.1 kg of magnesium stearate and mix well.
[0035] Particle size: mesh
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com