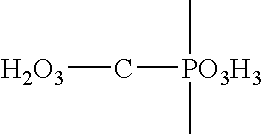Film coated tablet for improved upper gastrointestinal tract safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
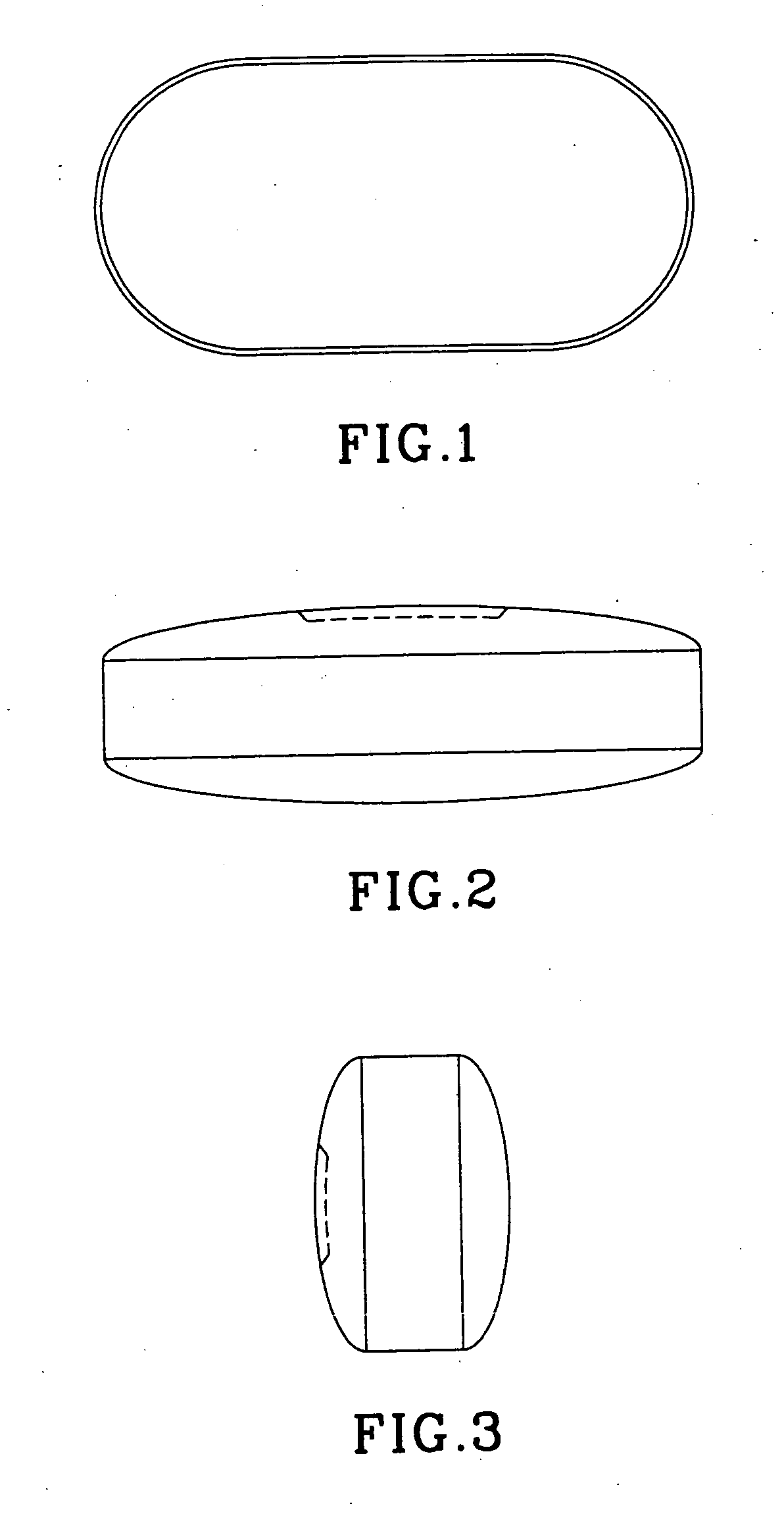
Modified Oval, Film-Coated Risedronate Tablet
[0063] The film-coating is applied to 110 kg of risedronate core tablets each weighing 240 mg.
Componentkg / batchmg / tabletRisedronate sodium tablets 30 mg110240Dri-Klear2.5985.67Chroma-Tone White0.7011.53Purified Water30.2kg65.9
[0064] Dri-Klear is a mixture of HPMC, HPC, polyethylene glycol, and silicon dioxide manufactured by Crompton and Knowles, Marwah, N.J., Chroma-Tone White is a mixture of HPC and titanium dioxide manufactured by Crompton and Knowles, Marwah, N.J.
[0065] The coating suspension is prepared as follows: [0066] 1. Add the Dri-Klear to hot purified water, 60-80° C., with agitation. [0067] 2. Cool the Dri-Klear solution to 40° C. or below, with continual mixing until all the Dri-Klear is dissolved. [0068] 3. Add the Chroma-Tone White to purified water with mixing. Disperse with the use of a high shear mixer for 10-25 minutes. [0069] 4. Add the pigment suspension (step 3) to the polymer solution (step 2) and mix. Continue...
example ii
Caplet Shaped, Film-Coated Alendronate Tablet
[0073] The film-coating is applied to 100 kg of alendronate core tablets each weighing 200 mg.
Componentkg / batchmg / tabletAlendronate sodium tablets 10 mg100200.0Opadry5.010.0Red iron oxide0.10.2Purified Water50kg100
[0074] Opadry is a commercial film-coating mixture manufactured by Colorcon, West Point, Pa. The coating suspension is prepared as follows: [0075] 1. Add the Opadry to room temperature purified water with agitation. [0076] 2. Mix until all the Opadry is dissolved. [0077] 3. Add the red iron oxide to purified water with mixing. Disperse with the use of a high shear mixer for 5 minutes. [0078] 4. Add the red iron oxide suspension (step 3) to the polymer solution (step 2) and mix.
[0079] Continue mixing until ready for use. [0080] 5. Load the core tablets into a 48 inch side vented coating pan. [0081] 6. Preheat the tablets until the exhaust temperature reaches approximately 40° C. and begin spraying. Apply the coating suspensio...
example iii
Oval Risedronate Tablets
[0083] The film-coated risedronate tablets are made by preparing granules containing the active, coating the granules, compressing into a tablet and then film-coating the tablets.
[0084] A. Preparation of the risedronate sodium granules, 212.5 kg
Componentkg / batchmg / g (Dry basis)Risedronate sodium2.511.7Lactose, anhydrous100471Microcrystalline cellulose100471Polyvinylpyrrolidone1047.1Purified Water75kg—
The granulation is prepared as follows: [0085] 1. Dissolve the polyvinylpyrrolidone in the purified water. [0086] 2. Mix the risedronate sodium, lactose and microcrystalline cellulose in a high shear mixer for 3 minutes. [0087] 3. Granulate the mixture with the polyvinylpyrrolidone solution with mixing over a 5 minute interval. [0088] 4. Dry the wetted mass in a fluid bed dryer at an inlet temperature of 60° C. [0089] 5. Mill the dried material using a hammer mill to achieve the desired granule size.
[0090] B. Coating of the granules and preparation of rised...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com