Supermolecular intercalation-structure slow-release captopril and its preparing method
A slow-release and slow-release agent technology, which is applied in the field of supramolecular intercalation structure slow-release captopril and its assembly, to achieve the effect of suppressing odor, good slow-release effect, and good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
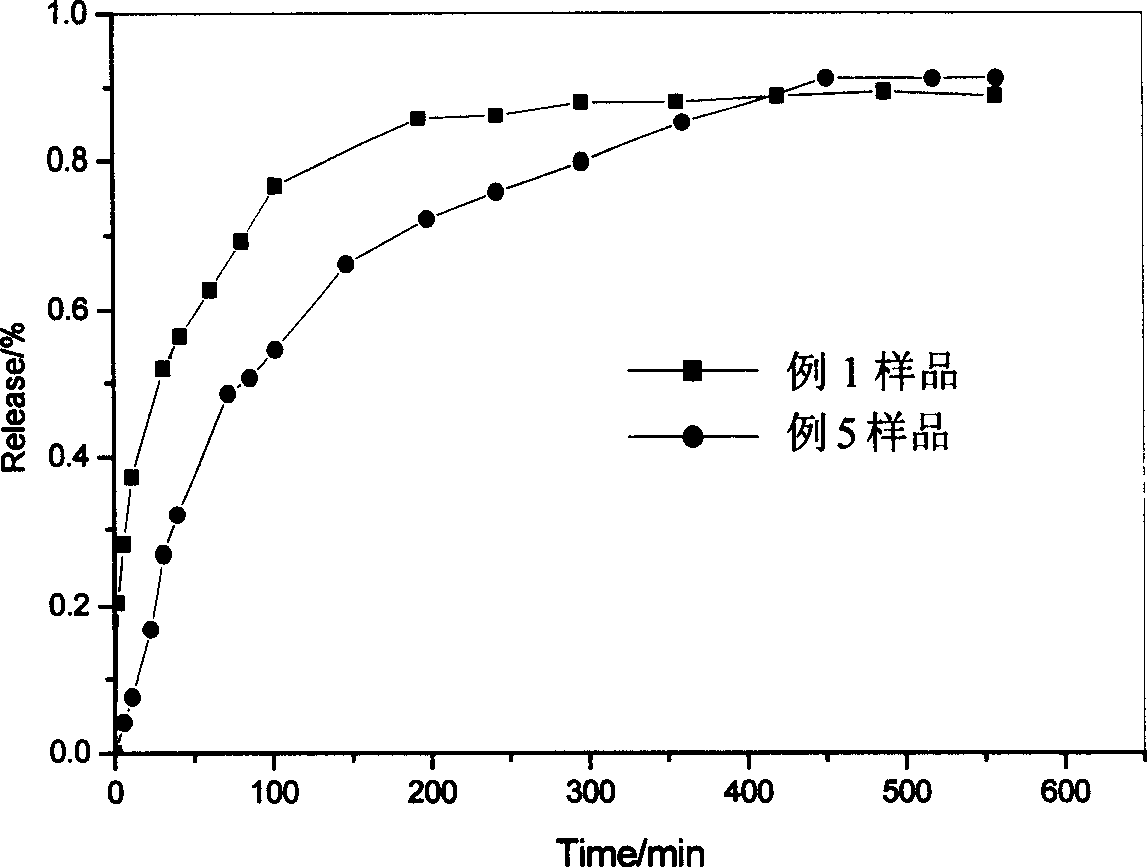
Embodiment 1
[0037] 1. Add Zn(NO 3 ) 2 ·6H 2 O(5.3545g) and Al(NO 3 ) 3 9H 2 O (2.2508g) is made into mixed salt solution A with Zn / Al molar ratio equal to 3 with 80ml water; In addition, NaOH (2.5000g) / captopril original drug (3.9114g) is made into molar ratio equal to 3 with 120ml water The mixed alkali solution B;
[0038] 2. In N 2 Under protection, slowly drop the mixed salt solution A into the vigorously stirred mixed alkali solution B, after the addition is complete, adjust the pH to 8.5 with 0.1M NaOH;
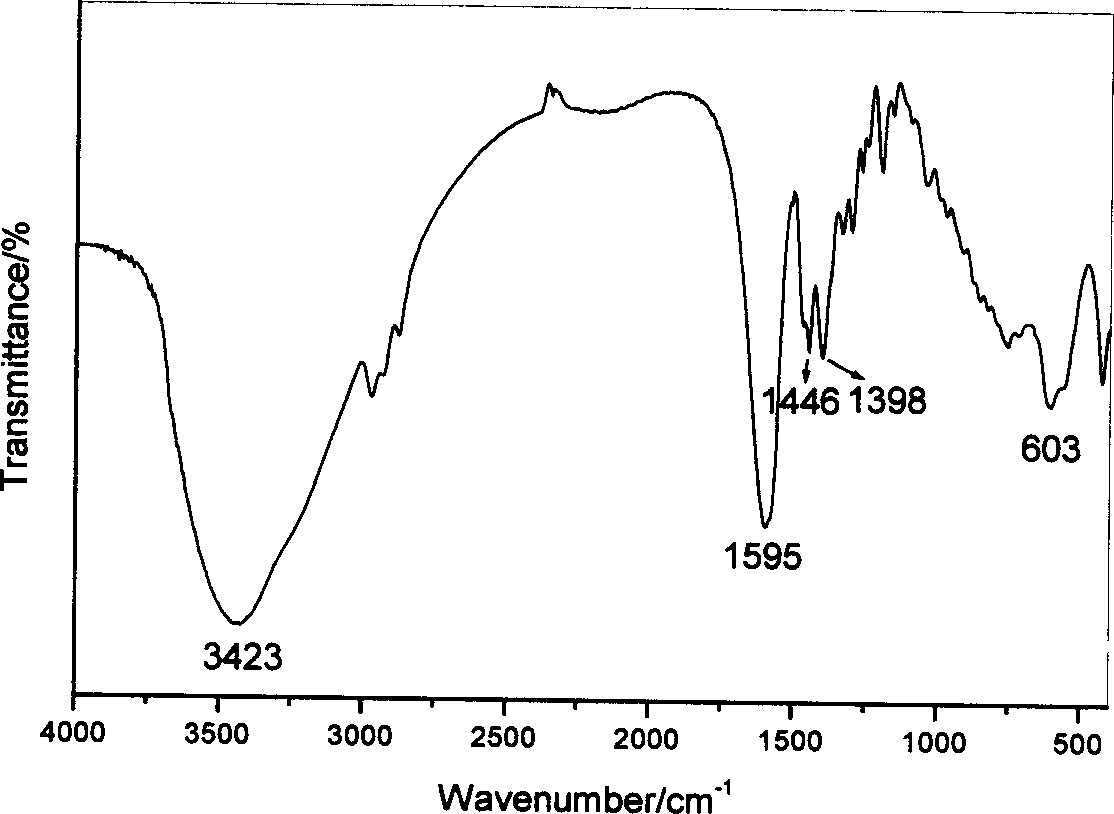
[0039] 3. The obtained slurry was crystallized at 25° C. for 48 hours, suction filtered, washed, and vacuum-dried at room temperature for 72 hours to obtain captopril-intercalated zinc-aluminum LDH. The water used in the process is decarbonized deionized water.
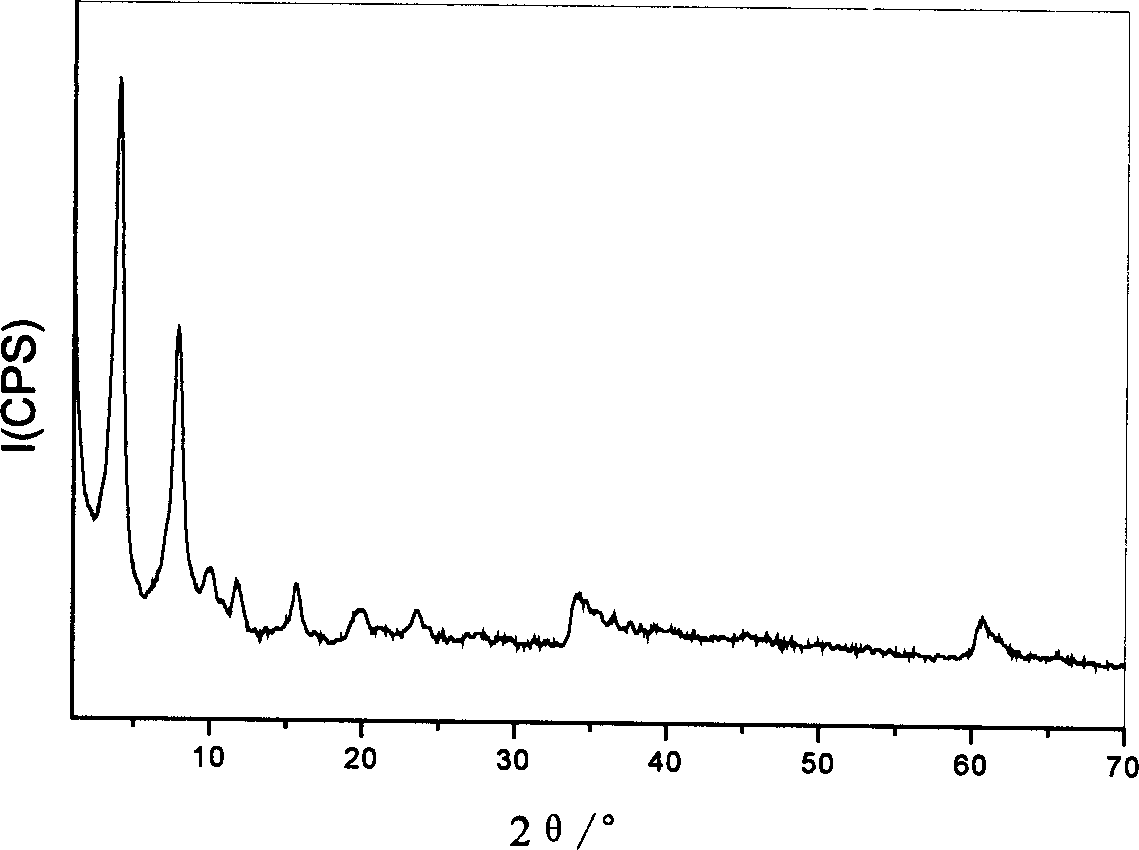
[0040] The obtained Cpl-LDHs were characterized by X-ray powder diffraction, the results are shown in figure 1 ,Depend on figure 1 It can be seen that the Cpl-LDHs has the crystal structure of hydrotalcite-like...
Embodiment 2
[0044] According to the synthesis process of Example 1, change step 2 to drop the mixed salt solution A and the mixed alkali solution B into deionized water at the same time, stir vigorously, control the pH to 9, and crystallize the obtained slurry at 25°C for 48 hours, and filter with suction , washed, and vacuum-dried at room temperature for 72 hours to obtain captopril-intercalated zinc-aluminum LDH. The water used in the process is decarbonized deionized water.
[0045] Adopt the method of embodiment 1 to analyze product, obtain its chemical formula / formation as:
[0046] [Zn 0.732 Al 0.268 (OH) 2 ](C 9 h 14 NO 3 S - ) 0.268 0.9H 2 O, captopril content is 33.2%, water content is 9.3%.
Embodiment 3
[0048] Zn(NO 3 ) 2 ·6H 2 O(35.6815g), Al(NO 3 ) 3 9H 2 O (22.6103g), NaNO 3 (9.0712g) is made into the mixed salt solution A that Zn / Al molar ratio is 3 with 160ml water, and NaOH (12.0282g) is made into alkaline solution B with 100ml water in addition, N 2 Under protection, slowly drop the alkaline solution B into the mixed salt solution A, stir vigorously, and stop the dropwise addition when the pH is 6.5. The slurry was crystallized at 70°C for 24 hours, then suction filtered, washed with water, and dried at 70°C for 18 hours to obtain zinc aluminum nitrate hydrotalcite.
[0049] The original drug of captopril (4.0000g) and the prepared zinc-aluminum nitrate hydrotalcite (3.0000g) were mixed in 100ml of water at a molar ratio of 4, and the pH was adjusted to 6.5 with 0.1M NaOH, and the resulting slurry was heated at 25°C After crystallization for 48 hours, suction filtration, washing, and vacuum drying at room temperature for 72 hours, the captopril-intercalated zinc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com