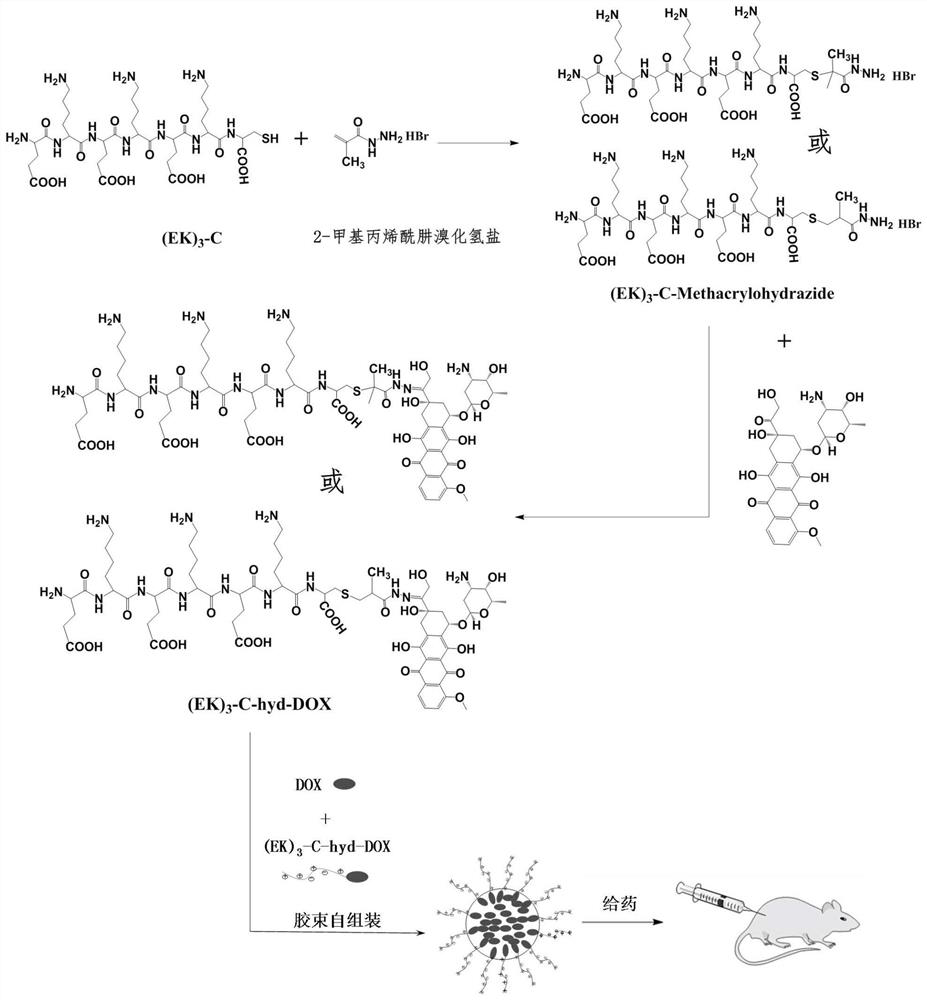
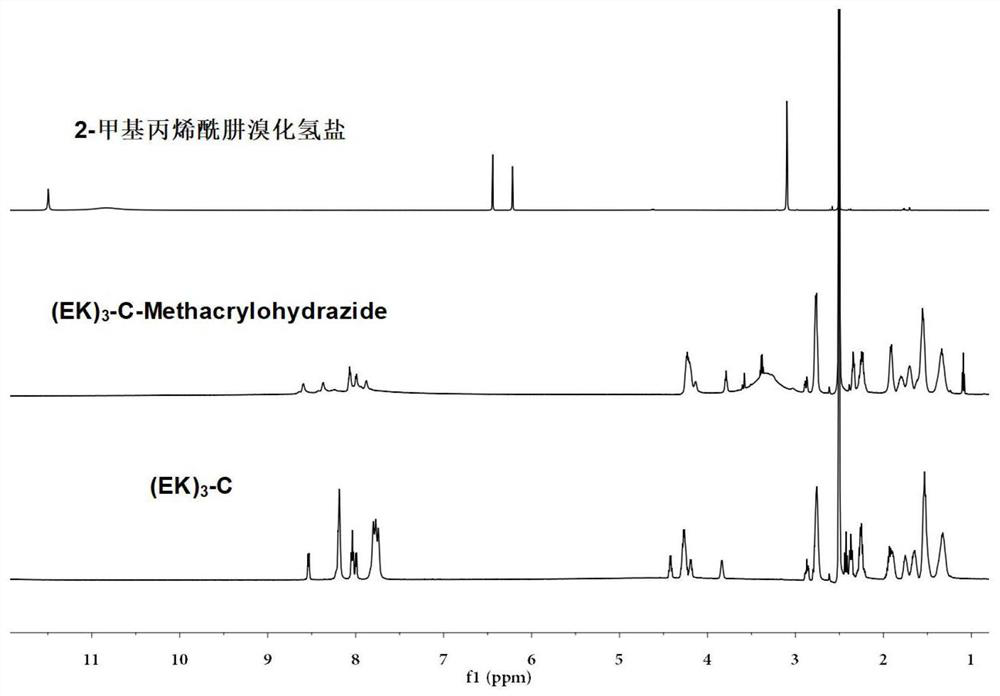
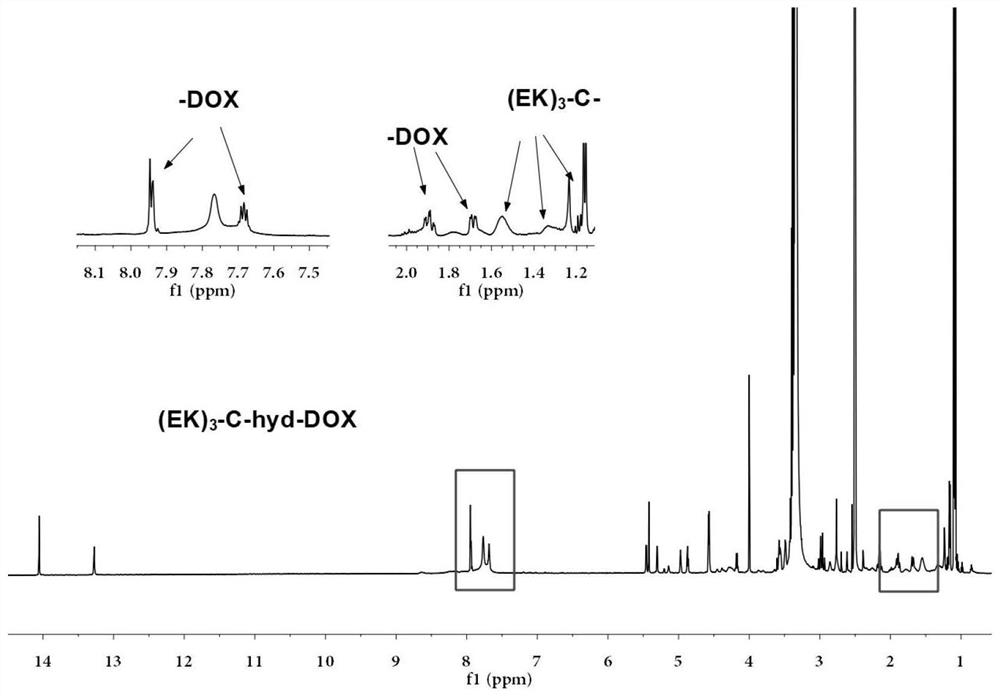
Sulfydryl-containing zwitterionic polypeptide modified doxorubicin derivative, nano-micelle and preparation methods of doxorubicin derivative and nano-micelle
A zwitterion and polypeptide modification technology, which is applied in the field of biomedicine, can solve the problems of low drug loading, low tumor targeting performance, and weak interaction of nano-drugs, achieve excellent anti-protein non-specific adsorption performance, and increase the enrichment amount , the effect of reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] This example provides a doxorubicin complex modified by a zwitterionic polypeptide, and nanomicelles formed by its self-assembly. The preparation steps are as follows: figure 1 As shown, specifically include:
[0065] (1) Use zwitterionic polypeptide (EK) composed of glutamic acid (E) and lysine (K) 3 -C, add 3mg (EK) per 1mL methanol 3 -C peptide configuration 3mg / mL (EK) 3 -C methanol solution. According to the thiol-containing zwitterionic polypeptide (EK) 3 The molar ratio of -C to 2-methacrylohydrazide hydrogen bromide is 1:2. Dissolve 6 mg of 2-methacrylohydrazide hydrogen bromide in 2 mL of methanol solution, add 5 mL of 3 mg / mL(EK) 3 -C methanol solution and stirred with nitrogen for 10 minutes, removed the nitrogen and sealed off the oxygen, then put it in a water bath at 50°C and stirred for 24 hours. Concentrate the reacted solution to 1 / 10 times the original volume, then add it to 15 mL of acetonitrile for recrystallization twice, wash and dry with et...
Embodiment 2
[0077] This embodiment provides a doxorubicin complex modified by a zwitterionic polypeptide, and nanomicelles formed by its self-assembly, specifically including:
[0078] (1) Use zwitterionic polypeptide (EK) composed of glutamic acid (E) and lysine (K) 1 -C, add 3mg (EK) per 1mL methanol 1 -C peptide configuration 3mg / mL (EK) 1 -C methanol solution. According to the thiol-containing zwitterionic polypeptide (EK)1 The molar ratio of -C to 2-methacrylohydrazide hydrogen bromide is 1:1.5, dissolve 9.5mg of 2-methacrylohydrazide hydrogen bromide in 2mL of methanol solution, add 5 mL of 3mg / mL(EK) 1 -C methanol solution, add 0.5 mg of 4-dimethylaminopyridine (DMAP) catalyst and stir for 10 min with nitrogen gas, remove nitrogen gas and seal with oxygen, place at room temperature at 25°C and irradiate and stir for 6 h under ultraviolet wavelength 365 nm. The reacted solution was concentrated to 1 / 10 of the original volume, then added to 10 mL of acetonitrile for recrystalliz...
Embodiment 3
[0083] This embodiment provides a doxorubicin complex modified by a zwitterionic polypeptide, and nanomicelles formed by its self-assembly, specifically including:
[0084] (1) Use zwitterionic polypeptide (EK) composed of glutamic acid (E) and lysine (K) 14 -C, add 3mg (EK) per 1mL methanol 14 -C peptide configuration 3mg / mL (EK) 14 -C methanol solution. According to the thiol-containing zwitterionic polypeptide (EK) 14 -C and 2-methacrylohydrazide hydrogen bromide molar ratio is 1:4, dissolve 3.3mg of 2-methacrylohydrazide hydrogen bromide in 2mL methanol solution, add 8.2mL 3mg / mL(EK) 14 -C methanol solution, add 1 mg of 4-dimethylaminopyridine (DMAP) catalyst and stir for 10 min with nitrogen gas, remove the nitrogen gas, isolate the oxygen, seal it, place it at room temperature and irradiate and stir for 12 h under ultraviolet wavelength 365nm. Concentrate the reacted solution to 1 / 10 of the original volume, then add it to 10 mL of acetonitrile for recrystallization...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com