Compound with hemicyanine-naphthalimide structure, and preparation method and application thereof
A naphthalimide and compound technology, which is applied in the field of compounds with a hemicyanine-naphthalimide structure, can solve the problems of poor biocompatibility of dyes, easy to be affected by biological internal environmental factors, and easy to produce cytotoxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A compound with half cyanine-naphthalimide structure, its structural formula is as follows:
[0030] .
[0031] The specific synthetic route of above-mentioned compound is referred to as follows:
[0032]
[0033] The synthesis steps are as follows:
[0034] (1) Synthesis of Compound 1
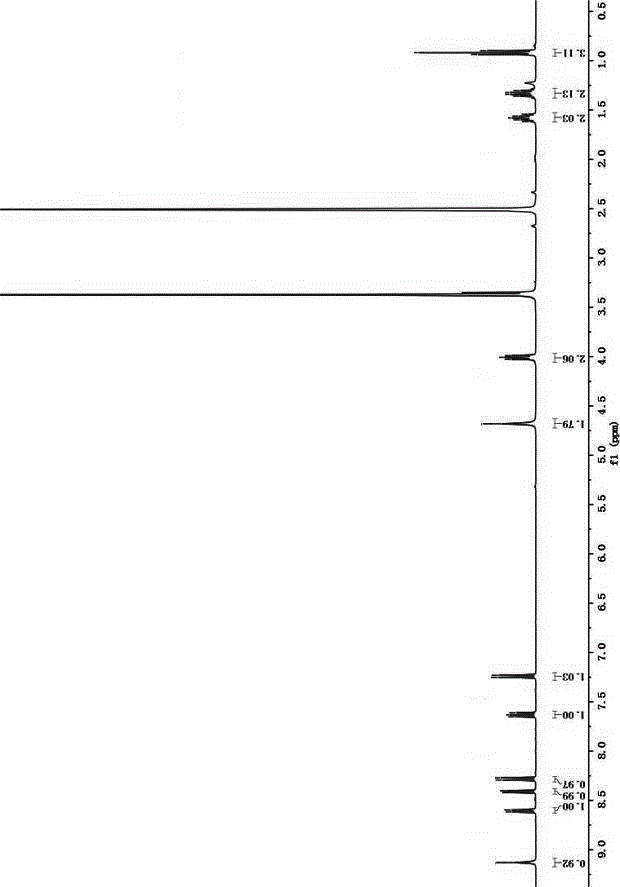
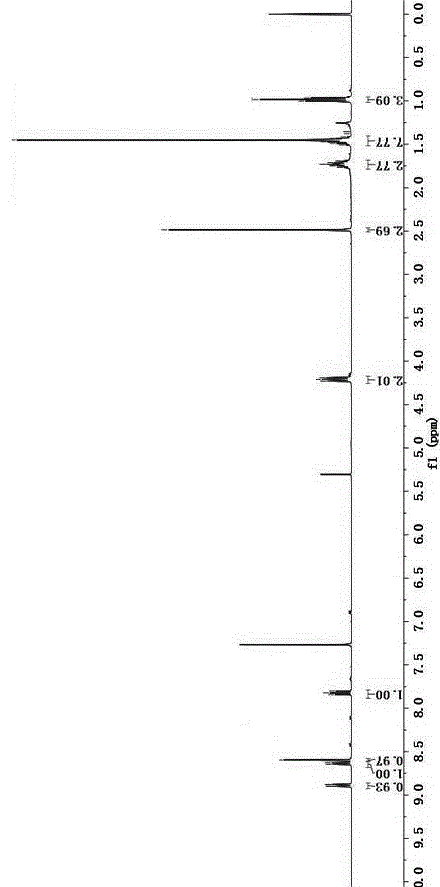
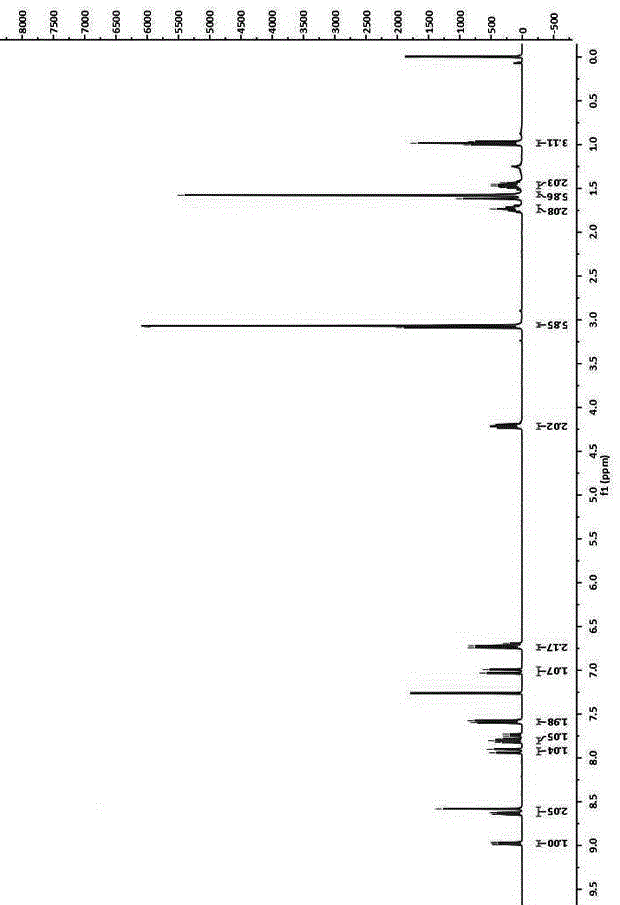
[0035] Add 4-bromo-1,8 naphthalene anhydride (5.54g, 20.0mmol) and n-butylamine (2.4mL, 24mmol) into a 500mL round bottom flask, add solvent ethanol 200mL, reflux for 12 hours under nitrogen protection, until the solution is clear, and depressurize Part of the solvent was distilled off and cooled to room temperature, and light yellow crystals were precipitated, which were recrystallized from ethanol to obtain light yellow crystals with a yield of 84.9%. Compound 1 1 See HNMR figure 1 , 1 HNMR (400MHz, CDCl 3 )δ: 8.65 (1H,d, J =7.3Hz),8.56(1H,d, J =8.5Hz),8.41(1H,d, J =7.9Hz),8.03(1H,d, J =7.9Hz),7.84(1H,t, J =7.9Hz),4.17(2H,t, J =7.5Hz),1.76–1.68(2H,m,),1.50–1.40(2H,m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com