Synthesis of two types of abiraterone derivatives
A technology of abiraterone and abiraterone ester, which is applied in the field of preparation of organic compounds, can solve the problems of enhanced toxicity and side effects, poor water solubility, insoluble, etc., and achieves the effect of low cost, easy operation and high-efficiency preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
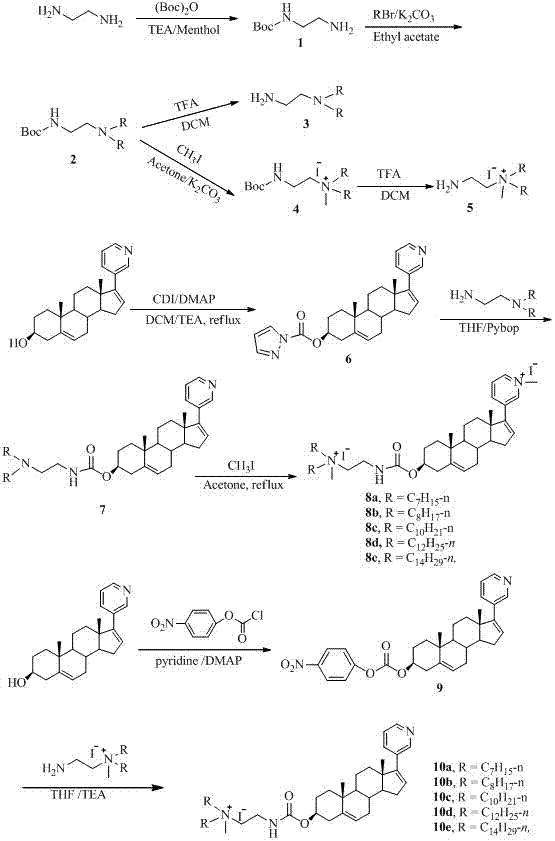
[0022] Example 1. β- N -methyl- N 1 , N 1 -Di-n-heptylcarbamate-Py- N - Preparation of Methyl-Abiraterone Ester Nanoparticles:
[0023]Add ethylenediamine (1.9 g, 33.3 mmol) into a 100.0 mL eggplant-shaped flask, dissolve it with methanol (50.0 mL), add triethylamine (10.1 g, 100.0 mmol) under stirring, and di-tert-butyl dicarbonate Esters (7.3 g, 33.3 mmol) were dissolved in methanol (60.0 mL), then added dropwise, after the addition was completed, the reaction was carried out at room temperature, TLC (V 乙酸乙酯 : V 甲醇 = 5:1) Monitor the reaction until no significant change. After concentration, a white solid precipitated, washed with water three times, filtered with suction, and concentrated the filtrate to obtain a yellow liquid compound (2-aminoethyl) tert-butyl carbamate (3.0 g, 56.6%). 1 H NMR (500 MHz, CDCl 3 ):δ (ppm) 5.01 (s, 1 H, CH 2 N H CO), 3.12-3.11 (d, 2 H, NHC H 2 CH 2 NH 2 ), 2.75-2.73 (m,2 H, NHCH 2 C H 2 NH 2 ), 1.39 (s, 9 H, (C H 3 ) 3 ...
Embodiment 2
[0029] Example 2. β- N -methyl- N 1 , N 1 -Dioctylcarbamate-Py- N - Preparation of Methyl-Abiraterone Ester Nanoparticles:
[0030] Add the compound (2-aminoethyl) tert-butyl carbamate (4.0 g, 25.0 mmol) into a 100.0 mL round bottom flask, dissolve it in ethyl acetate, add anhydrous K under stirring 2 CO 3 (13.8 g, 100.0 mmol), 1-bromooctane (19.3 g, 100.0 mmol ). The reaction mixture was at 70 o C under reflux reaction 48 h, TLC (V 石油醚 : V 乙酸乙酯 = 5 : 1) Monitor the reaction until the basic reaction of the starting material is complete. Filter and concentrate. by column chromatography (eluent: V 石油醚 : V 乙酸乙酯 = 5 : 1) separated and purified to obtain a white solid (2-( N 1 , N 1 -Dioctyl)ethyl)carbamate (6.1 g, 58.9%). 1 H NMR (500 MHz, CDCl 3 ): δ (ppm) 5.0 (s, 1 H, N H CH 2 CH 2 ), 3.13-3.12 (d, 2 H, NHC H 2 CH 2 ),2.48-2.46 (m, 2 H, NHCH 2 C H 2 ), 2.38-2.35 (m, 4 H, N(C H 2 CH 2 ) 2 ), 1.43 (s, 9 H, (C H 3 ) 3 C), 1.39-1.37 (m, 4 H, N(CH...
Embodiment 3
[0034] Example 3. β- N -methyl- N 1 , N 1 -Didecylcarbamate-Py- N - Preparation of Methyl-Abiraterone Ester Nanoparticles:
[0035] Add the compound imidazole carbamate abiraterone (4.0 g, 25.0 mmol) into a 100.0 mL round bottom flask, dissolve it in ethyl acetate, add anhydrous K under stirring 2 CO 3 (13.8 g, 100.0 mmol), 1-bromodecane (22.1 g, 100.0 mmol ). The reaction mixture was at 70 o C under reflux for 48 h, with TLC (V 石油醚 : V 乙酸乙酯 = 5 : 1) Monitor until the raw material no longer changes. Filter and concentrate. by column chromatography (eluent: V 石油醚 : V 乙酸乙酯 = 5 : 1) Separated and purified to obtain a white solid (2-( N 1 , N 1 -didecyl)ethyl)carbamate (1.0 g, 61.8%). 1 H NMR (500 MHz, CDCl 3 ): δ (ppm) 5.0 (s, 1 H N H CH 2 CH 2 ), 3.14-3.13 (d, 2 H NHC H 2 CH 2 ), 2.49-2.47(m, 2 H, NHCH 2 C H 2 ), 2.39-2.36 (m, 4 H, N(C H2 CH 2 ) 2 ), 1.44 (s, 9 H, (C H 3 ) 3 C),1.40-1.38 (m, 4 H, N(CH 2 C H 2 ) 2 ), 1.30-1.26 (d, 36 H, 2 C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com