Synthetic method of silodosin
A technology of silodosin and compounds, applied in the field of pharmaceutical chemical synthesis, can solve problems such as spraying materials, violent reactions, unfavorable process control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
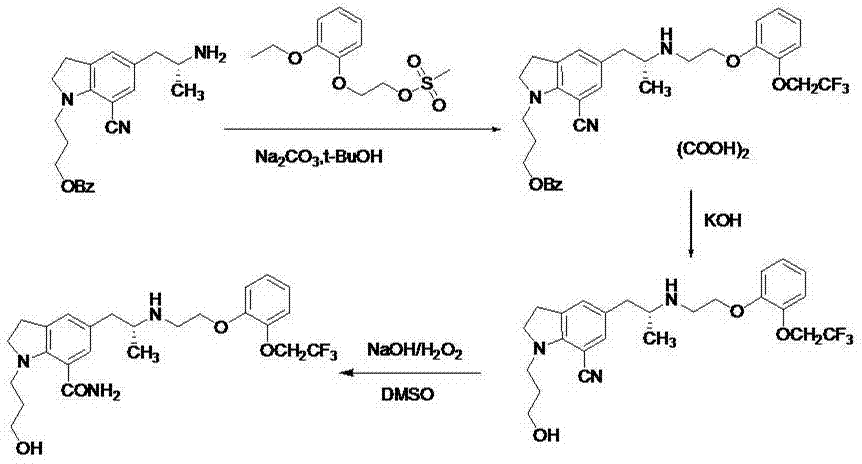
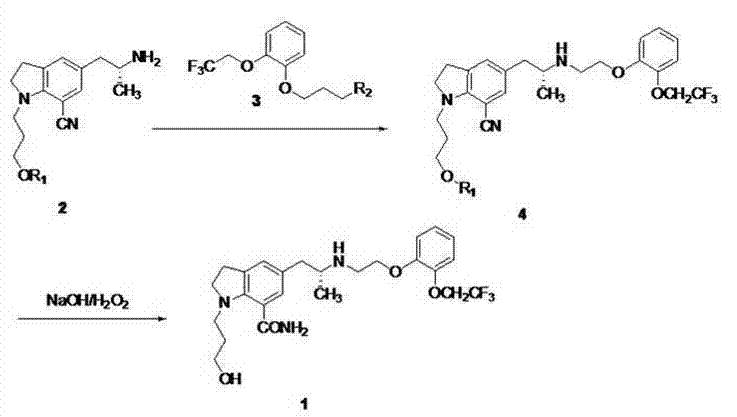
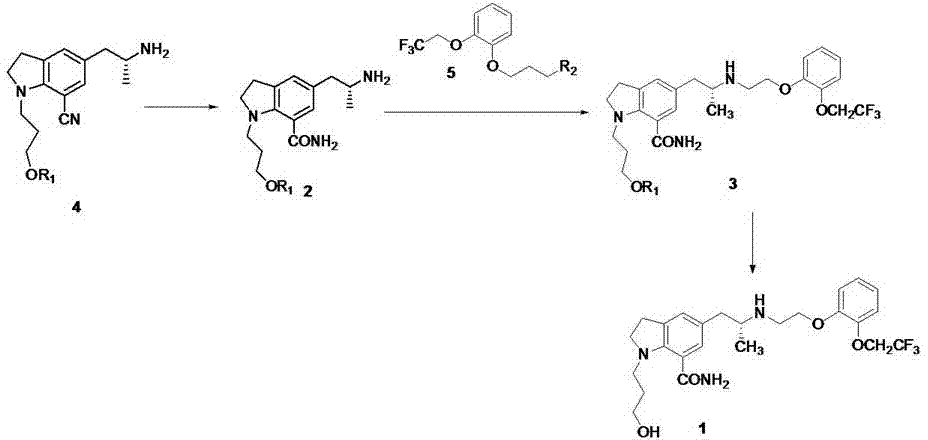
[0034]To a mixture of ethyl acetate (500 mL) and aqueous potassium carbonate (133 g) (500 mL) was added 5-[(2R)-2-aminopropyl]-2,3-dihydro-1-[3-(benzyl] Acyloxy)propyl]-1H-indole-7-carbonitrile-mono-tartrate (100g), stirred at room temperature for 1 hour, separated the ethyl acetate layer, the aqueous layer was extracted once with 500mL of ethyl acetate, combined with ethyl acetate The ester layer was washed once with 300 mL of saturated potassium carbonate solution and once with 300 mL of saturated brine, dried over anhydrous sodium sulfate, filtered and spin-dried. Add 451g polyphosphoric acid and 44g benzoic acid to the concentrate, heat up to 85°C, stir for about 5h, cool to room temperature, add 500mL ice water and 400mL ethyl acetate, carefully add 470g anhydrous sodium carbonate in stages, separate out organic phase, the aqueous phase was extracted once with ethyl acetate (300 mL), the organic phases were combined, back-extracted with 1 mol / L hydrochloric acid (100 mL x...
Embodiment 2
[0038] To a mixture of ethyl acetate (50 mL) and potassium carbonate (7.6 g) aqueous solution (50 mL) was added 5-[(2R)-2-aminopropyl]-2,3-dihydro-1-[3-(benzene Formyloxy)propyl]-1H-indole-7-carbonitrile-mono-tartrate (2.8g), stirred at room temperature for 30min, separated the ethyl acetate layer, the aqueous layer was extracted once with 50mL of ethyl acetate, combined with acetic acid The ethyl ester layer was washed once with 50 mL of saturated potassium carbonate solution and once with 50 mL of saturated brine, dried over anhydrous sodium sulfate, filtered and spin-dried. 6 mL of methanesulfonic acid was added to the concentrate, the temperature was raised to 93 °C, stirred for about 4 h, cooled to room temperature, added with 50 mL of ice water, adjusted to pH=3 with sodium carbonate, the aqueous layer was extracted with ethyl acetate (20 mLⅹ3), the organic phase was Discarded, the aqueous layer was adjusted to pH=9 with sodium carbonate solution, extracted with dichloro...
Embodiment 3
[0042] Add 5-[(2R)-2-aminopropyl]-2,3-dihydro-1-[3-(benzoyloxy)propyl]-1H-indole- 1.2 g of 7-carboxamide, 1.22 g of 2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl bromide, 367 mg of sodium carbonate, 10 mL of tert-butanol, react at 90°C for about 24 hours, The reaction solution was concentrated to dryness under reduced pressure, 15 mL of dichloromethane was added, stirred and filtered, the filter cake was washed twice with 5 mL of dichloromethane, the filtrate was washed with 0.05N aqueous phosphoric acid (15 mLⅹ3), the organic phase was discarded, and the dichloromethane used for the aqueous phase was Extraction (15mL), the organic phase was discarded, the aqueous layer was adjusted to pH=9 with sodium carbonate, extracted with butyl acetate (20mLⅹ2); the organic phases were combined, washed with 0.25N aqueous ammonium dihydrogen phosphate (20mLⅹ2), saturated brine Washed (15 mL), dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain 3-(7-carboxamide-5-((2R)-2-(2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com