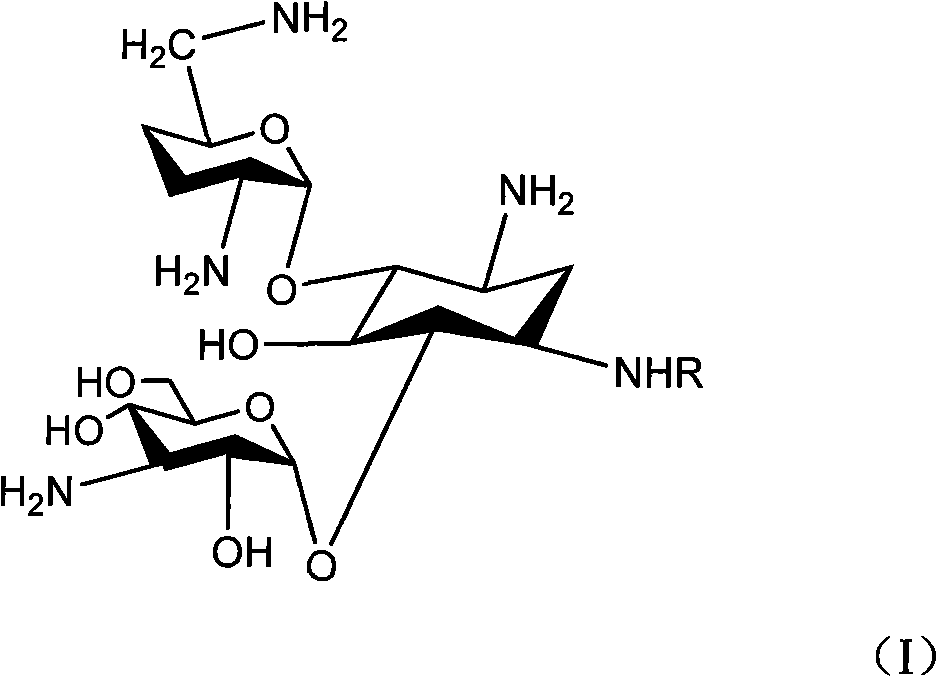
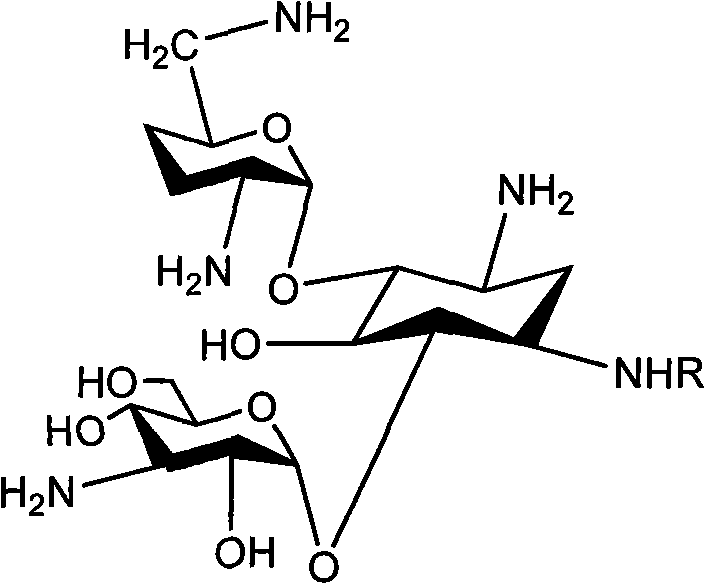
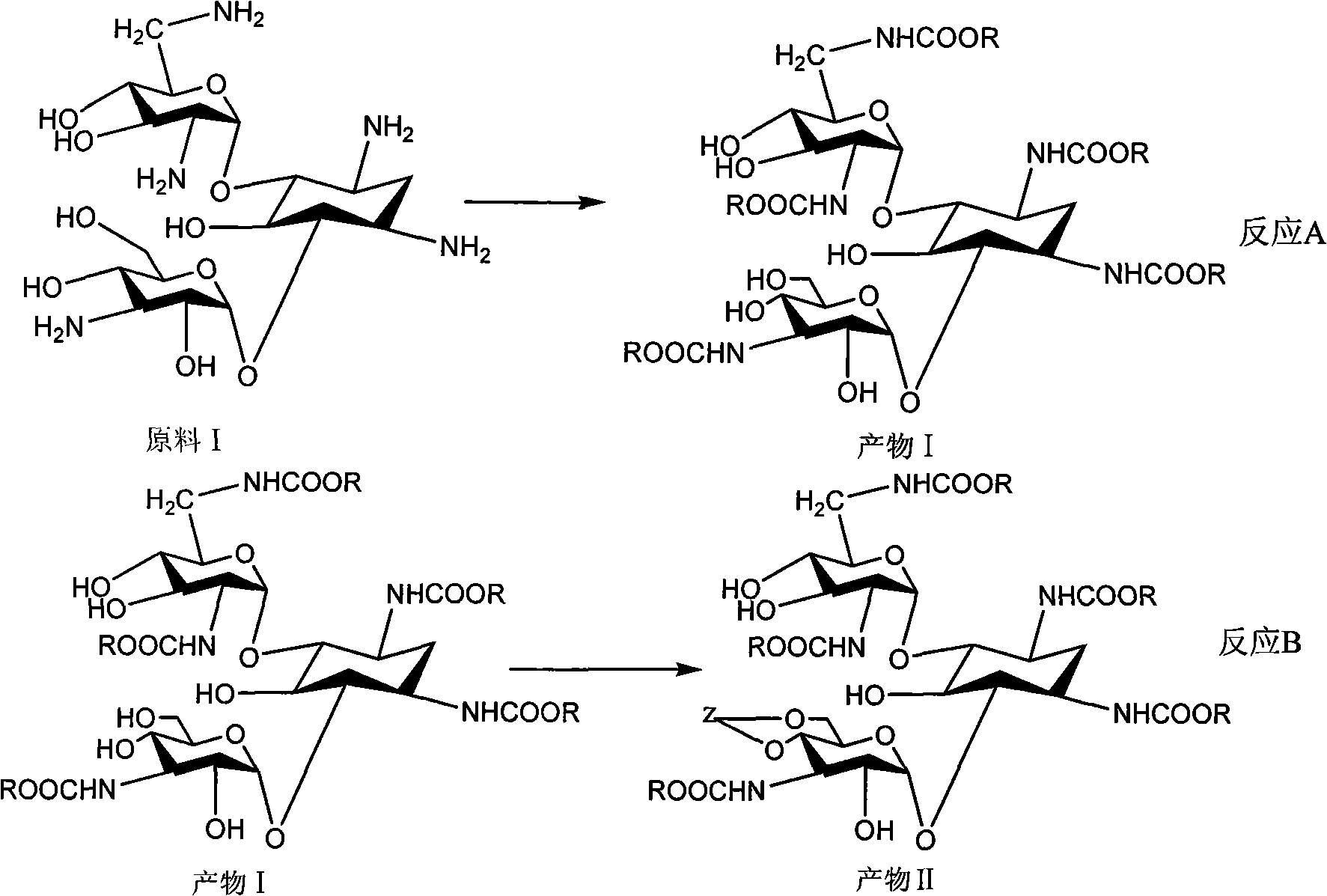
Method for synthesizing Arbekacin and intermediate dibekacin thereof
A synthetic method, the technology of dibekacin, applied in the field of organic synthesis, can solve the problems of difficult post-processing, affecting yield, increasing reaction cost, etc., and achieve the effect of reducing the difficulty of reaction operation, facilitating industrial production, and reducing reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] (1) Weigh 4.84g of kanamycin B and 8.6g of anhydrous p-toluenesulfonic acid into 48mL of DMF, stir for 0.5 hours, then measure 20.2mL of 1,1-dimethoxycyclohexane into the above In the reaction system, pump it with water for 1 hour every 12 hours, react at 25°C for 24 hours, adjust the reaction solution to neutrality with triethylamine, concentrate, add water, pass through macroporous weakly acidic acrylic cation exchange resin (HD-2 type) (Shanghai Huazhen Science and Technology Co., Ltd.) to obtain 4″, 6″-oxo-cyclohexylidene kanamycin B 5.2g with a yield of 92%.
[0083] (2) Weigh 2.81g of dried 4″, 6″-oxo-cyclohexylidene kanamycin B, dissolve it in 100mL of pyridine, cool the system to 0°C, weigh 7.6g of benzylsulfonyl chloride and 4.9 Put all the g DMAP into the reaction system, react for 2 hours, add 2 mL of water to terminate the reaction, concentrate, add chloroform to dissolve, wash with water, and concentrate again to obtain 1,3,2′,6′,3″-penta-N- Benzylsulfonyl...
Embodiment 2
[0094] When the reaction temperature in step (1) was 45°C, 2.0 g of 4", 6"-oxo-cyclohexylidene kanamycin B was obtained with a yield of 35%. Other steps are with embodiment 1.
Embodiment 3
[0096] When the reaction temperature in step (1) was 60° C., 1.5 g of 4″, 6″-oxo-cyclohexylidene kanamycin B was obtained with a yield of 27%. Other steps are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com