Synthesis method of tetracarboxylic dianhydride with fluorinated rigid structure
A technology of tetracarboxylic dianhydride and rigid structure, which is applied in organic chemistry and other fields, and can solve problems such as bone and tooth deformity and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
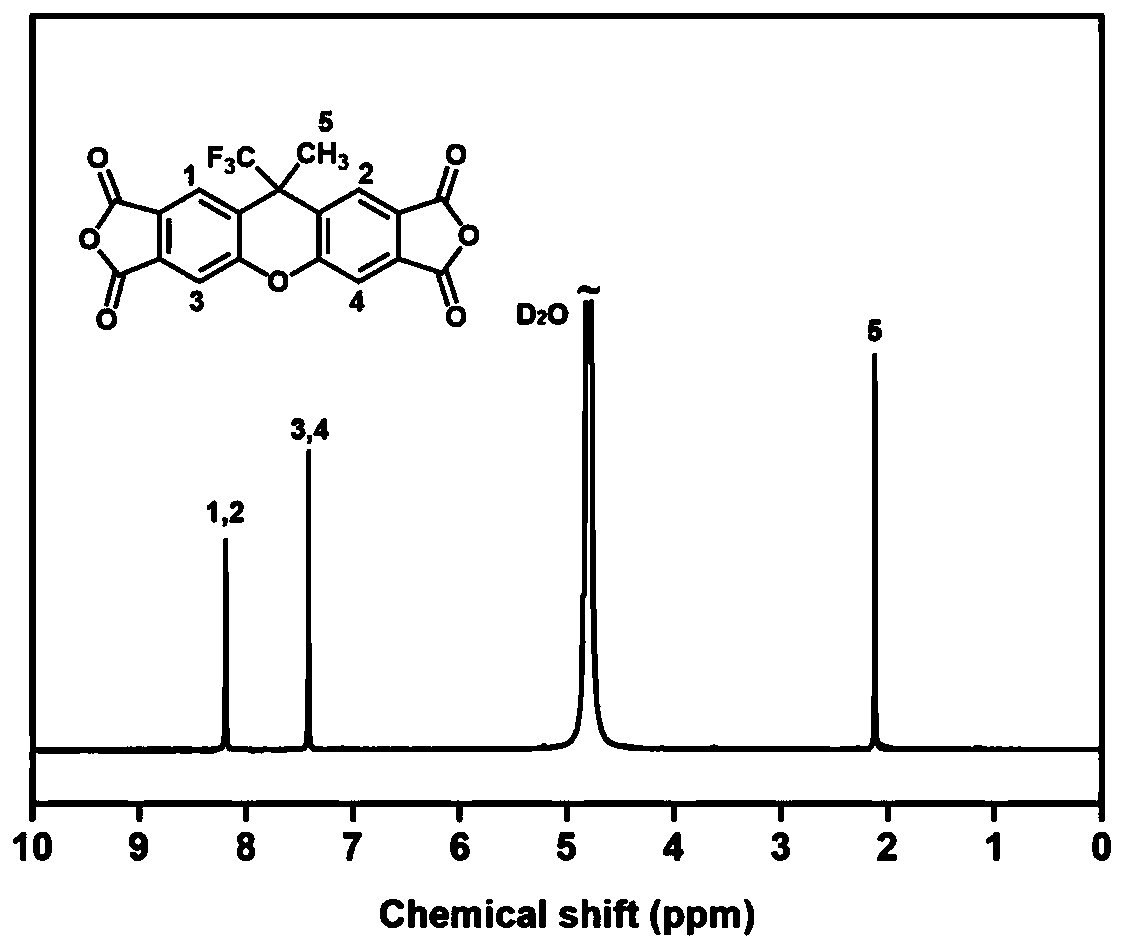
[0041] A kind of tetracarboxylic dianhydride with fluorinated rigid structure, its structural formula is as follows:
[0042]
[0043] The tetracarboxylic dianhydride synthetic method shown in this structural formula comprises:
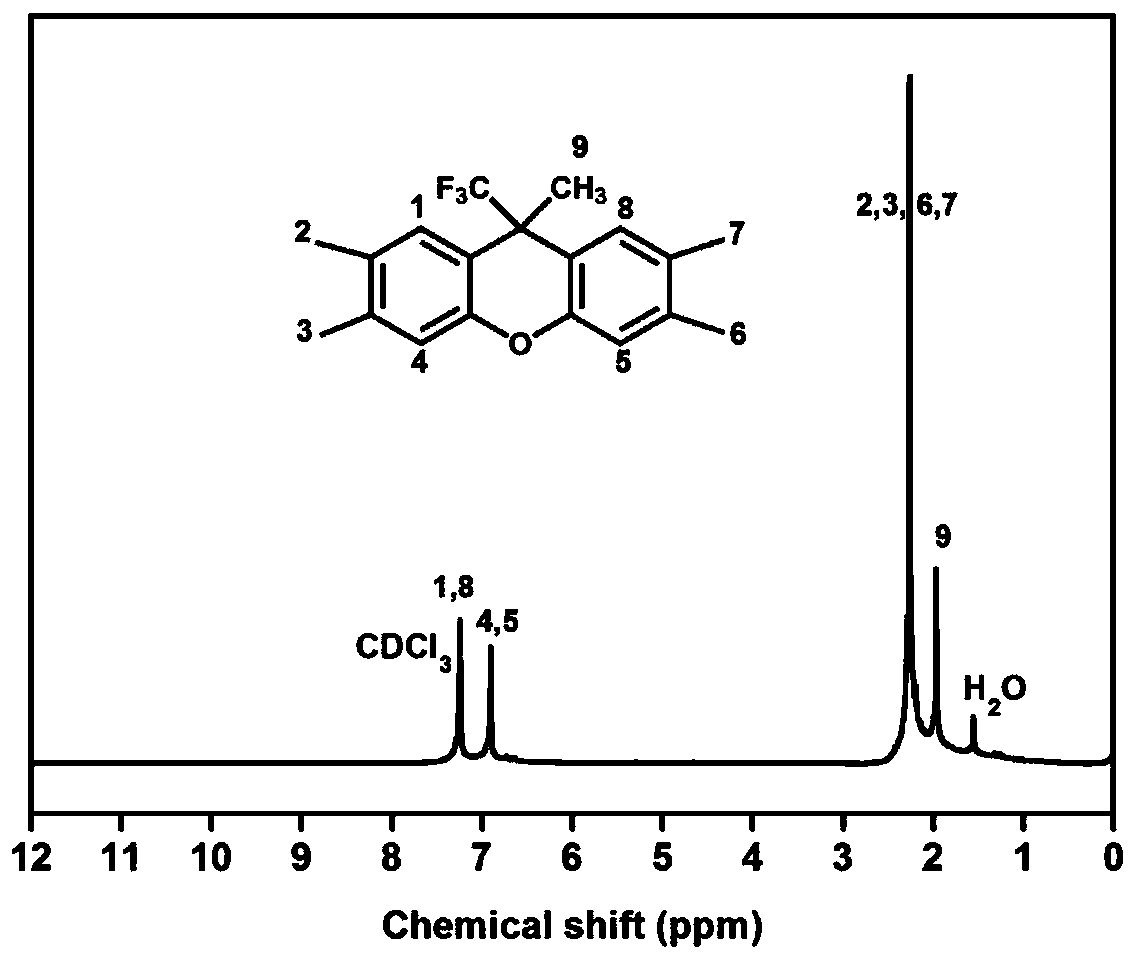
[0044] S1. Under nitrogen atmosphere and ice bath conditions, add 30g 1,1,1-trifluoroacetone (0.268mol) into 350mL dichloromethane and stir until completely dissolved, then add 66g 3,4-dimethylphenol ( 0.536mol) and 40g trifluoromethanesulfonic acid (0.268mol) were stirred until completely dissolved, stirred and reacted at room temperature for 24 hours, after the reaction was finished, the reaction solution was slowly poured into deionized water, the organic phase was extracted and rotary evaporated to obtain a solid, vacuum Dry overnight to obtain 38g 9-methyl-9-(trifluoromethyl)-2,3,6,7-tetramethyloxanthene (yield: 44%), and its hydrogen spectrogram refers to figure 1 shown;
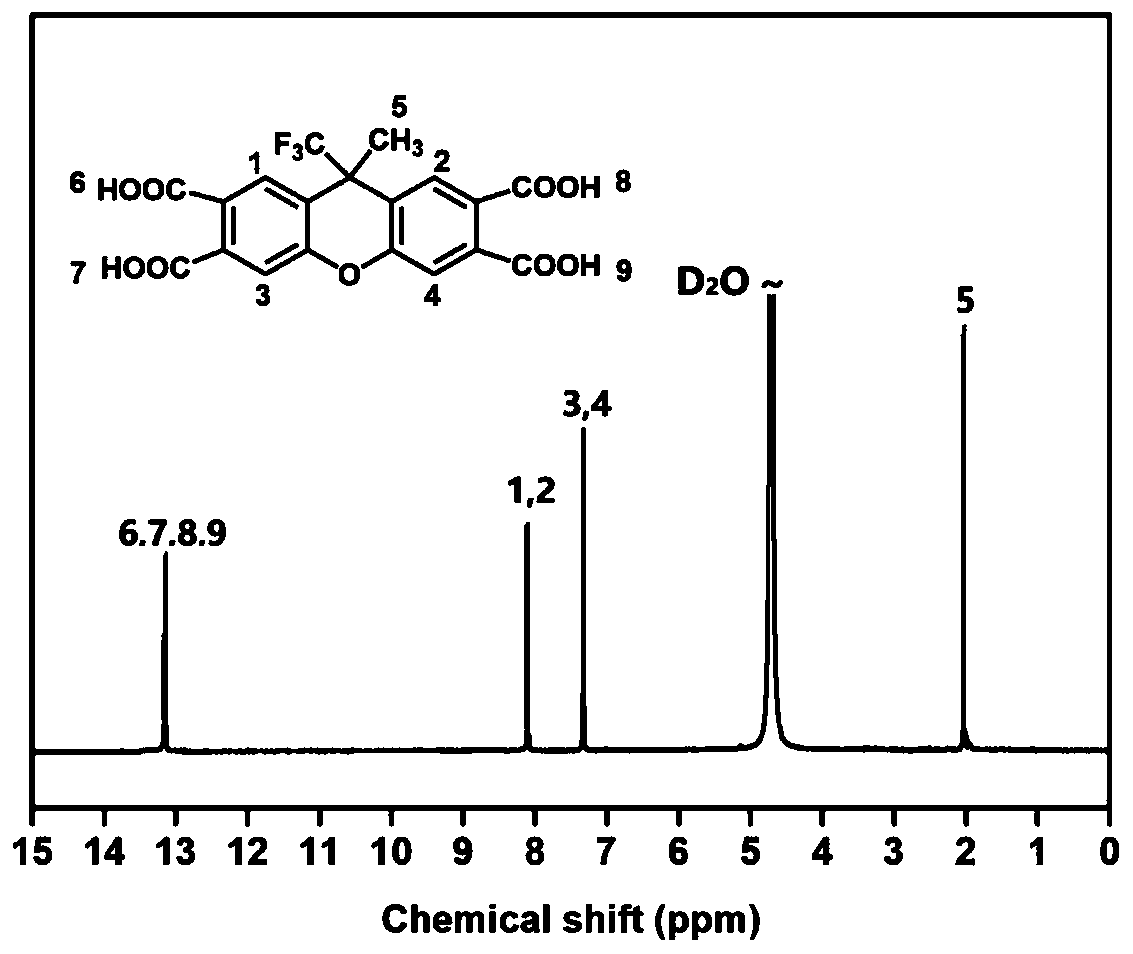
[0045] S2, 20g 9-methyl-9-(trifluoromethyl)-2,3,6,7-tetramethylxanth...
Embodiment 2
[0048] A kind of tetracarboxylic dianhydride with fluorinated rigid structure, its structural formula is as follows:
[0049]
[0050] The tetracarboxylic dianhydride synthetic method shown in this structural formula comprises:
[0051]S1. Under nitrogen atmosphere and ice bath conditions, add 30g of hexafluoroacetone (0.181mol) into 350mL of dichloromethane and stir until completely dissolved, then add 44g of 3,4-dimethylphenol (0.362mol) and 28g of tris Fluoromethanesulfonic acid (0.181mol) was stirred until it was completely dissolved, and the reaction was stirred at room temperature for 24 hours. After the reaction, the reaction solution was slowly poured into deionized water, and the organic phase was extracted and rotary evaporated to obtain a solid, which was dried overnight in vacuum to obtain 24 g of 9 - bis(trifluoromethyl)-2,3,6,7-tetramethylxanthene (yield: 36%);
[0052] S2. Add 20g of 9-bis(trifluoromethyl)-2,3,6,7-tetramethylxanthene (0.053mol) to a mixture ...
Embodiment 3
[0055] A kind of tetracarboxylic dianhydride with fluorinated rigid structure, its structural formula is as follows:
[0056]
[0057] The tetracarboxylic dianhydride synthetic method shown in this structural formula comprises:
[0058] S1. Under nitrogen atmosphere and ice bath conditions, add 30g 2,2,2-trifluoroacetophenone (0.172mol) into 350mL dichloromethane and stir until completely dissolved, then add 42g 3,4-dimethyl Phenol (0.344mol) and 20g trifluoroacetic acid (0.172mol) were stirred until completely dissolved, stirred and reacted at room temperature for 24 hours, after the reaction was completed, the reaction solution was slowly poured into deionized water, the organic phase was extracted and rotary evaporated to obtain a solid, vacuum Dry overnight to obtain 43 g of 9-phenyl-9-(trifluoromethyl)-2,3,6,7-tetramethylxanthene (yield: 64%);
[0059] S2, 20g 9-phenyl-9-(trifluoromethyl)-2,3,6,7-tetramethylxanthene (0.053mol) was added to a mixture of pyridine:water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com