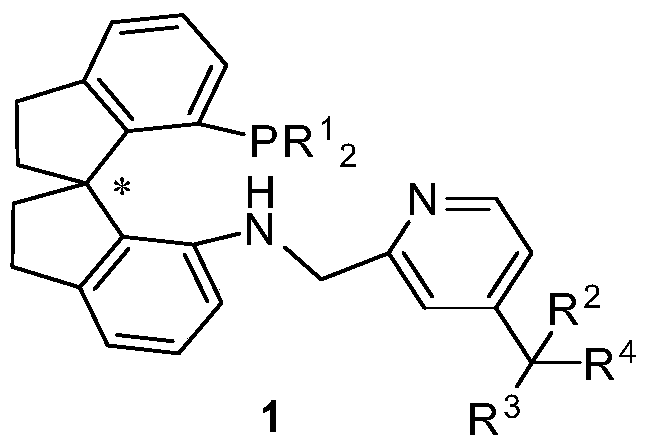
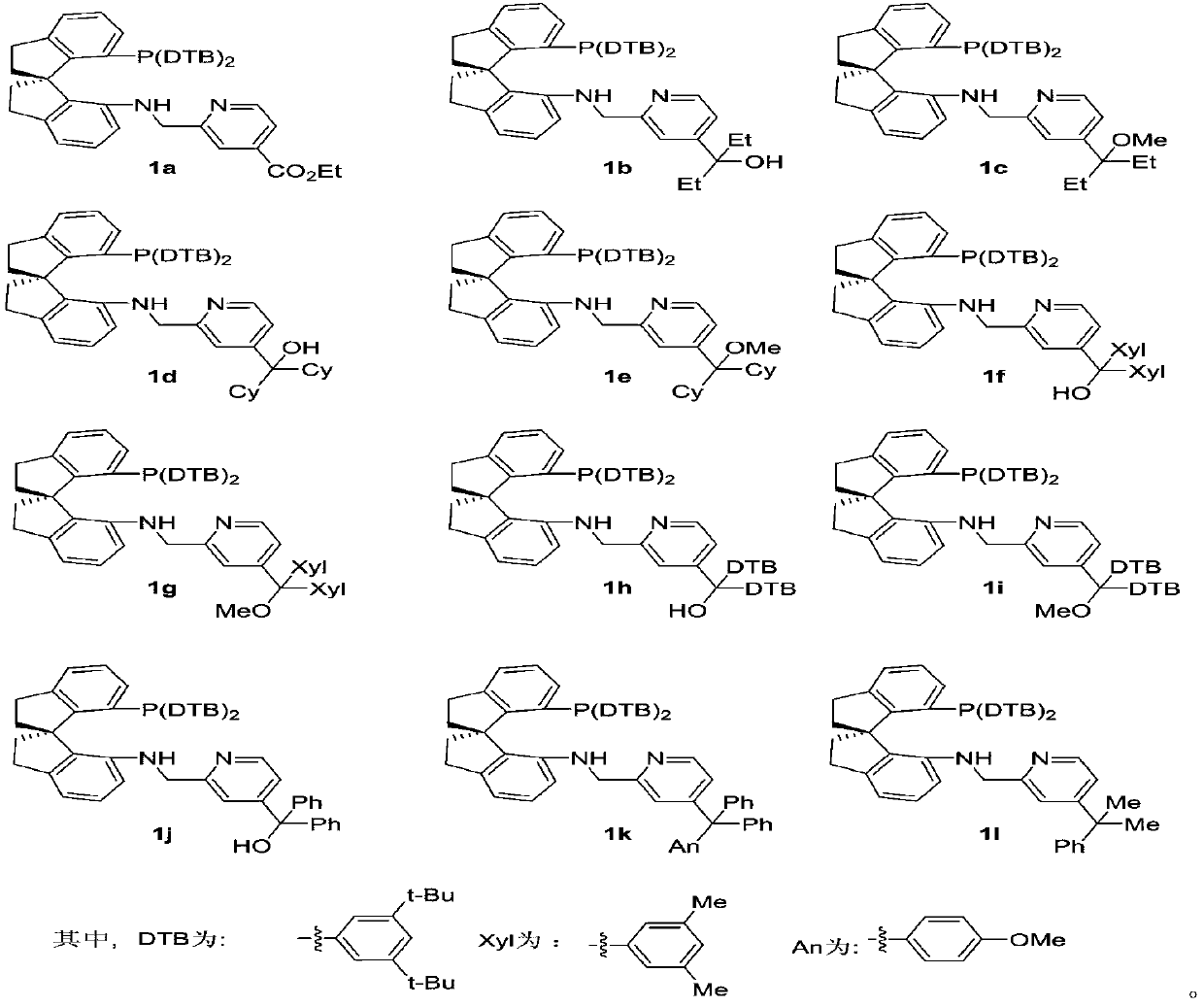
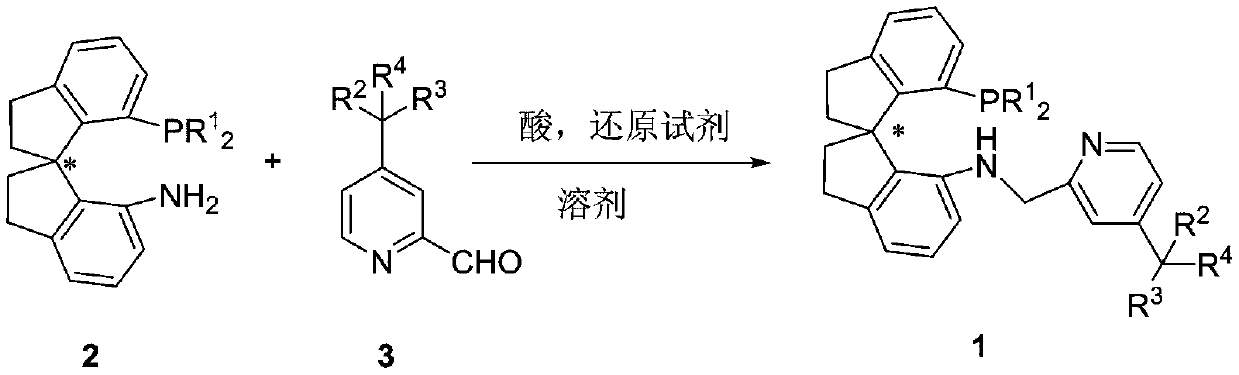
Preparation method of 4-position substituted chiral spirocyclic aminophosphine ligand on pyridine ring and application thereof
A spirocyclic amino and pyridine ring technology is applied in the field of preparation of chiral spirocyclic aminopyridine tridentate ligands, which can solve problems such as low enantioselectivity, and achieve strong adjustment ability, mild conditions and high catalytic activity. and the effect of the chirality-inducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] In an argon atmosphere, weigh (R)-7'-bis-(3,5-di-tert-butylphenyl)phosphino-7'-amino-1,1'-spirodihydroindane SpiroAP (160mg, 0.25mmol) into a 100mL dry Schlenk tube, inject 30mL of anhydrous methanol into a syringe, and stir to dissolve. 4-Carboxylic acid ethyl pyridinecarbaldehyde (89.5mg0.50mmol) and glacial acetic acid (45mg, 0.75mmol) were added dropwise. The reaction was stirred at room temperature for 2 hours. Open the anti-port plug and pour NaBH at one time 3 CN (31.5mg, 0.50mmol), the reaction was carried out at 40°C for 12 hours. Cool to room temperature after the reaction, spin the system to dryness, add ethyl acetate to dissolve, and quench with saturated sodium bicarbonate solution. Extract with ethyl acetate, combine the organic phases, dry the organic phases with anhydrous magnesium sulfate, remove the desiccant by suction filtration, and remove the solvent from the filtrate with a rotary evaporator. The residue was subjected to silica gel...
Embodiment 2
[0041]
[0042] The operation process is the same as in Example 1a. White solid 164mg, 80% yield. Melting point: 99-100°C. 1 H NMR (400MHz, CDCl 3 )δ: 8.23(d, J=4.8Hz, 1H), 7.32(d, J=7.2Hz, 1H), 7.24(s, 1H), 7.22–7.17(m, 2H), 7.12–7.05(m, 2H ), 7.02(d, J=4.4Hz, 1H), 6.91(s, 1H), 6.85(d, J=6.8Hz, 2H), 6.76–6.65(m, 3H), 6.11(d, J=8.0Hz ,1H),4.19(br,1H),4.00(dd,J=16.0,6.8Hz,1H),3.57(d,J=16.0Hz,1H),3.11–2.82(m,4H),2.55–2.42( m,1H),2.25–2.17(m,2H),2.16–2.07(m,1H),1.73–1.66(m,4H),1.14(s,18H),1.07(s,18H),0.70–0.60( m,6H). 13 C NMR (101MHz, CDCl 3 )δ158.5, 155.3(2), 152.7, 150.0(2), 149.9(2), 148.7, 144.5, 144.3, 144.2, 144.1, 144.1, 138.5, 138.4, 136.1, 136.0, 135.0, 134.8, 134.1(2), 133. ,133.1,128.4,128.2,128.1,128.0(2),126.9,125.8,122.3,121.5,119.0,117.6,114.0,108.9,77.1,67.2,61.9,61.8,48.4,39.0,38.9,35.92,34.8 ,34.7,31.4,31.3,31.0,29.9,7.8,7.7. 31 P NMR (162MHz, CDCl 3 )δ:–18.41. HRMS (MALDI) Calcd for C 56 h 74 N 2 OP([M+H] + ):821.5533; Found: 821.5538.
Embodiment 3
[0044]
[0045] The operation process is the same as in Example 1a. White solid 166mg, 80% yield. Melting point: 96-97°C. 1 H NMR (400MHz, CDCl 3 )δ: 8.22(d, J=5.2Hz, 1H), 7.32(d, J=7.2Hz, 1H), 7.24(s, 1H), 7.22–7.17(m, 2H), 7.12–7.04(m, 2H ),7.04–7.01(m,1H),6.93(s,1H),6.85(dd,J=8.0,1.6Hz,2H),6.72(dd,J=7.6,1.6Hz,2H),6.68(d, J=7.6Hz, 1H), 6.09(d, J=7.6Hz, 1H), 4.25(d, J=4.4Hz, 1H), 4.04–3.93(m, 1H), 3.56(dd, J=16.0, 2.4 Hz,1H),3.09–3.03(m,1H),3.01(s,3H),2.98–2.85(m,2H),2.54–2.44(m,1H),2.26–2.17(m,2H),2.15– 2.08(m,1H),1.82–1.72(m,2H),1.67–1.60(m,2H),1.14(s,18H),1.07(s,18H),0.65–0.56(m,6H). 13 C NMR (101MHz, CDCl 3 )δ158.3,153.9,152.9,152.7,149.9(2),148.6,144.4,144.3,144.2,144.1,144.1,138.6,138.5,136.2,136.1,134.9,134.7,134.1(2),1301.2,1283. ,128.2,128.1,128.0,127.9,126.9,125.8,122.2,121.4,119.8,118.7,113.9,108.9,81.0,61.9,61.8,49.5,48.4,39.0(2),35.9,35.0(2),34.8(2 ), 34.6, 31.4, 31.0, 28.3, 7.4(2). 31 P NMR (162MHz, CDCl 3 )δ:-19.00. HRMS (MALDI) Calcd for C 57...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com