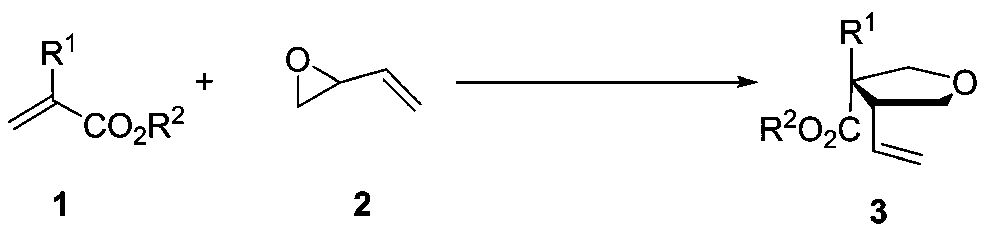
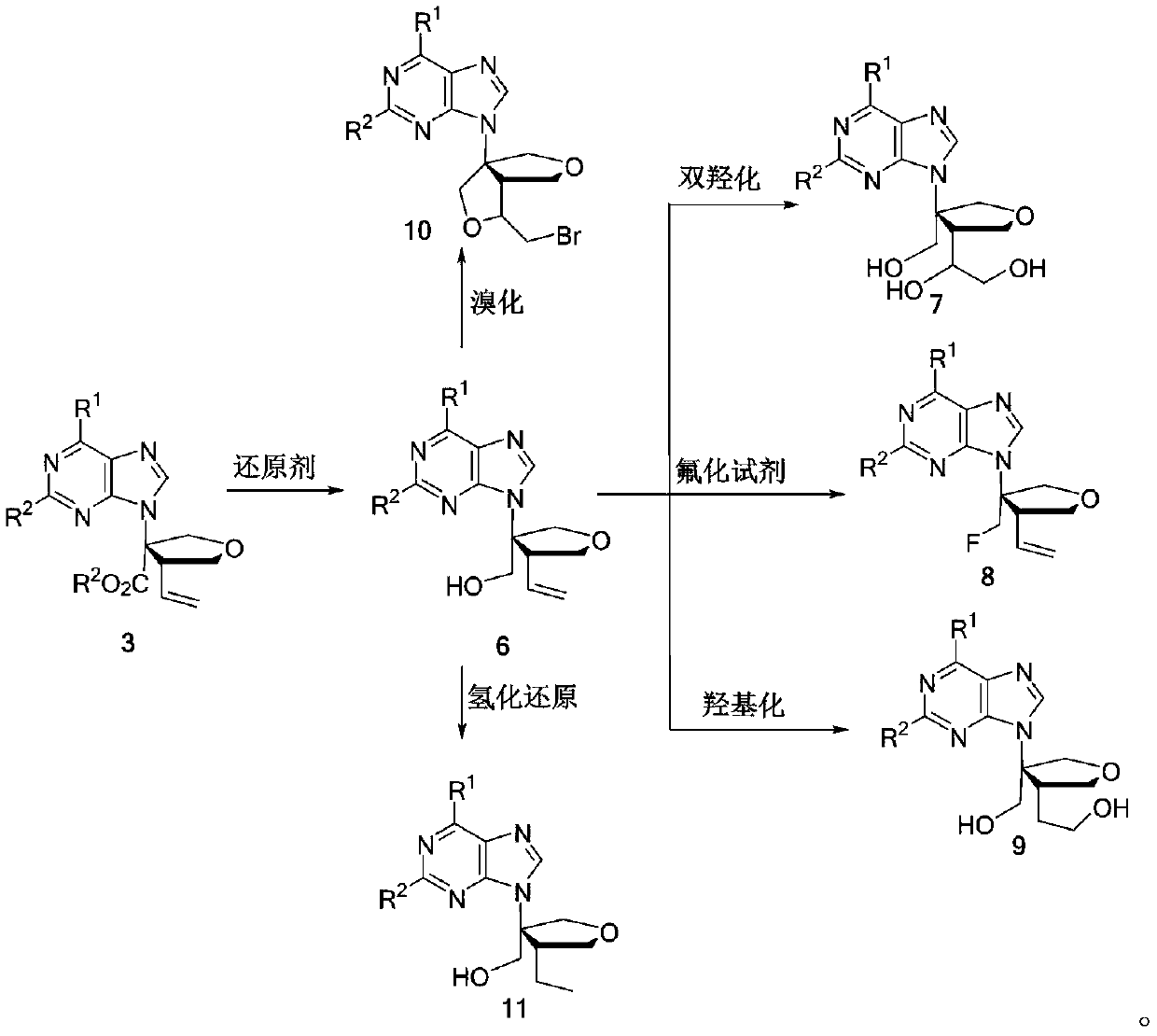
Method for synthesizing chiral isonucleoside analogues through asymmetric cycloaddition reaction
An asymmetric and isonucleoside technology, which is applied in the field of asymmetric cycloaddition synthesis of chiral isonucleosides and chiral isonucleosides, can solve the problem of low yield, high cost, and difficult preparation of chiral substrates and other problems, to achieve the effect of efficient synthesis method, easy access to reaction raw materials, and rich product structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027]
[0028]
[0029] a Unless otherwise specified, the steps of the reaction are as follows: under nitrogen atmosphere, metal (10mol%), ligand (12mol%), 1a (0.1mmol), 2 (0.12mmol) were reacted for 1 day in a solvent. b Separation yield. c The dr value of the crude product was tested by NMR. d The ee value was separated by high performance liquid chromatography.
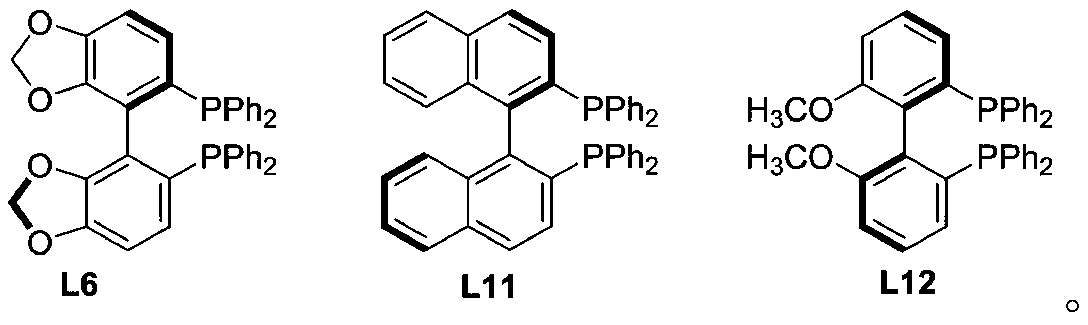
[0030] During the screening of reaction conditions, the effect of ligand on the reaction (label 1-14), the effect of reaction solvent on the reaction (label 15-18), and the influence of temperature on the reaction (label 19-21) were investigated. Pd(PPh 3 ) 4 is the best metal, ligand L6 is the best ligand, and toluene is the best solvent.
[0031] Investigation of reaction conditions: In a 10mL vacuum tube, add α-5-methyluracil substituted methyl acrylate 1a (31.4mg, 0.1mmol), Pd(PPh 3 ) 4 (5.8mg, 5mol%) and L6 (3.6mg, 6mol%). Nitrogen was replaced 3 times, then 0.5 mL of dichloromethane...
Embodiment 2
[0036] In a 10mL vacuum tube, add α-5-ethyluracil substituted methyl acrylate 1b (32.8mg, 0.1mmol), Pd(PPh 3 ) 4 (5.7mg, 5mol%) and L6 (3.7mg, 6mol%). Nitrogen was replaced 3 times, then 0.5 mL of toluene was added and stirred for half an hour, then epoxybutene 2 (9.0 mg, 0.12 mmol) was dissolved in 0.5 mL of toluene and added to the reaction tube. The reaction tube was left to react at room temperature for 1 day. Track the reaction with TLC, after terminating the reaction, add dichloromethane / water for extraction, dry the organic phase over anhydrous sodium sulfate, concentrate the organic phase in vacuo, then obtain the target compound 3b through column chromatography, the yield is 90%, 6:1dr and 92% ee.
Embodiment 3
[0038] In a 10mL vacuum tube, add α-5-fluorouracil substituted methyl acrylate 1c (32.8mg, 0.1mmol), Pd(PPh 3 ) 4(11.4 mg, 10 mol%) and L6 (7.4 mg, 12 mol%). Nitrogen was replaced 3 times, then 0.5 mL of toluene was added and stirred for half an hour, then epoxybutene 2 (9.0 mg, 0.12 mmol) was dissolved in 0.5 mL of toluene and added to the reaction tube. The reaction tube was left to react at room temperature for 1 day. Track the reaction with TLC, after terminating the reaction, add dichloromethane / water for extraction, dry the organic phase over anhydrous sodium sulfate, concentrate the organic phase in vacuo, then obtain the target compound 3c through column chromatography, the yield is 81%, 6:1dr and 91% ee. HPLC CHIRALCEL IA, n-hexane / isopropanol=90 / 10, flow rate 0.8mL / min, column temperature 25°C, wavelength 250nm, retention time: 34.007min(major), 37.667min(minor). 1 H NMR (600MHz, CDCl 3 ): δ7.90(d, J=7.8Hz, 2H), 7.73(d, J=6.6Hz, 1H), 7.69-7.67(m, 1H), 7.53-7.50(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com