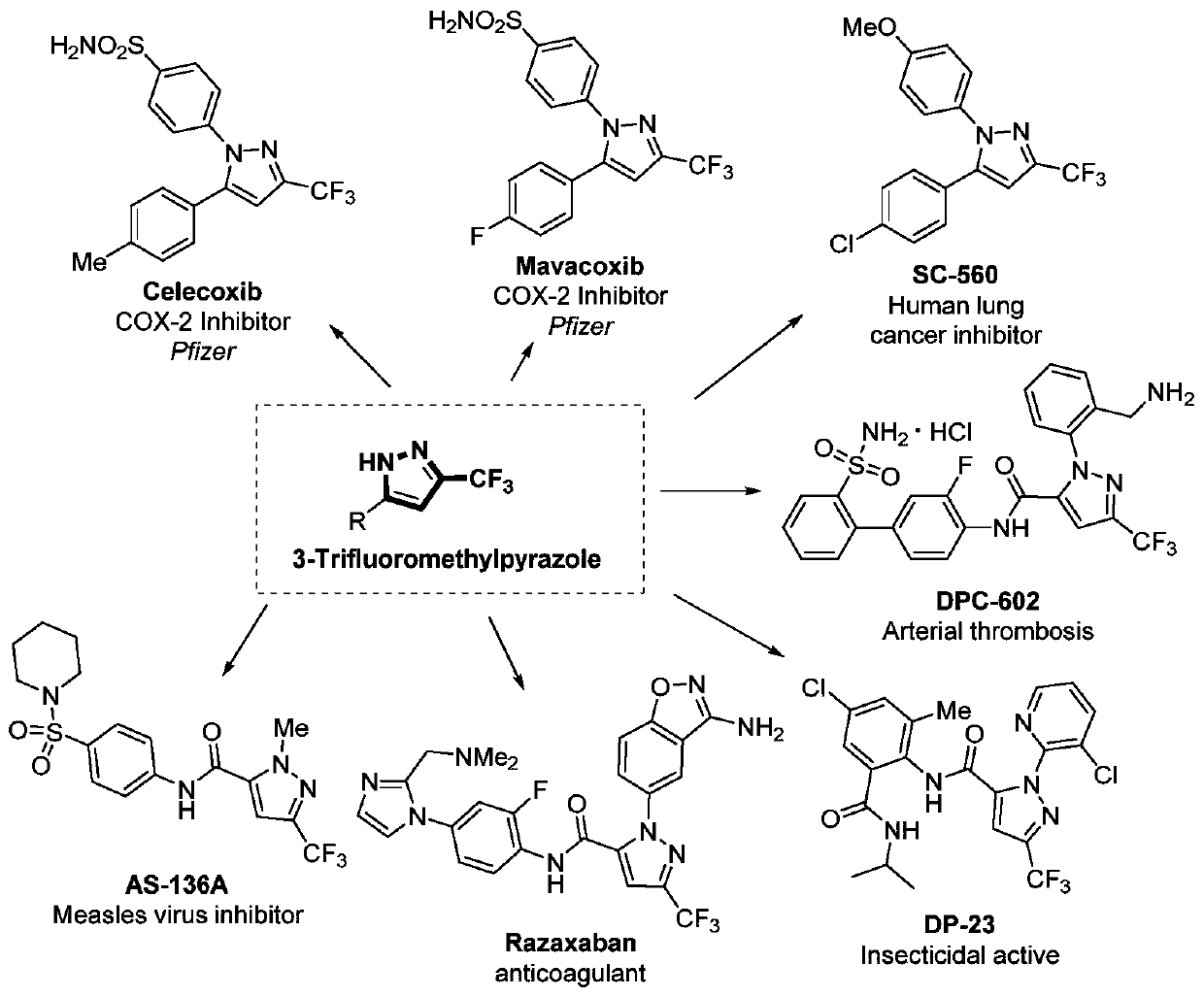
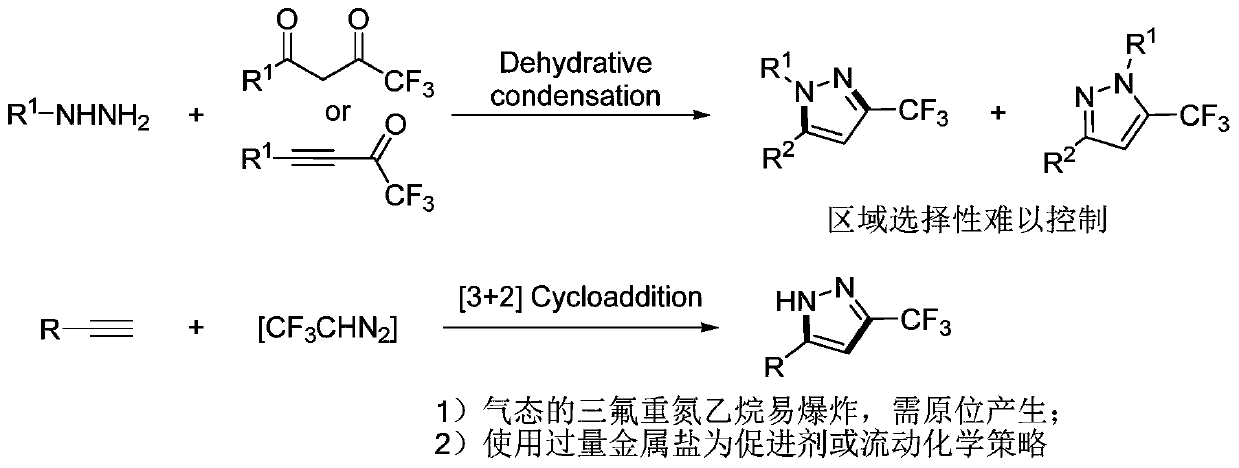
3-trifluoromethyl substituted pyrazole derivative and synthesis method thereof
A technology of pyrazole derivatives and trifluoromethyl, applied in the field of 3-trifluoromethyl substituted pyrazole derivatives and synthesis thereof, can solve problems such as unfavorable amplification synthesis, achieve good industrial application prospects, efficient synthesis methods, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
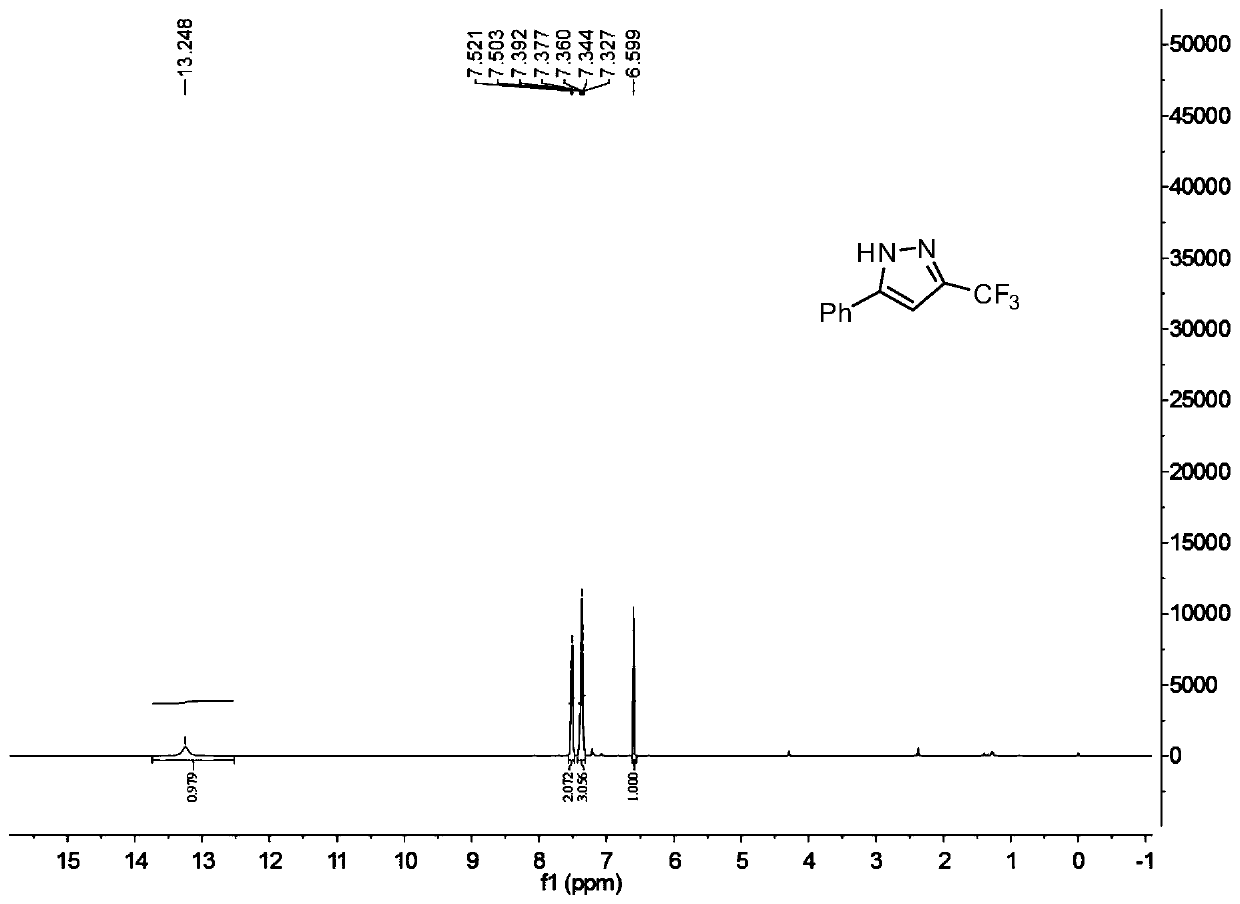
[0057] This example provides a 3-trifluoromethyl-substituted pyrazole derivative and a preparation method thereof.
[0058] Its preparation method is as follows: in an air atmosphere, in a 25 ml reaction flask equipped with a reflux condenser, add 1 mmol of benzaldehyde, 1.2 mmol of p-toluenesulfonyl hydrazide, 6 ml of toluene, 3 mmol of 1,8- Diazabicycloundec-7-ene, 2 mmol 2-bromo-3,3,3-trifluoropropene, the reaction system was stirred and reacted at 60°C for 6 hours, the heating and stirring were stopped, and water, ethyl acetate Ester extraction reaction solution, the ethyl acetate layer is subjected to vacuum rotary evaporation, solvent removal, and then separated and purified by column chromatography to obtain the target product, the column chromatography eluent used is petroleum ether and ethyl acetate, and the volume ratio is 10:1; the yield of product was 88%.
Embodiment 2
[0060] This example provides a 3-trifluoromethyl-substituted pyrazole derivative and a preparation method thereof.
[0061] Its preparation method is as follows: in an air atmosphere, in a 25 ml reaction flask equipped with a reflux condenser, add 1 mmol of benzaldehyde, 1.2 mmol of benzenesulfonyl hydrazide, 6 ml of toluene, 3 mmol of 1,8-bis Azabicycloundec-7-ene, 2 mmol 2-bromo-3,3,3-trifluoropropene, the reaction system was stirred and reacted at 60°C for 6 hours, stopped heating and stirring, added water, ethyl acetate The reaction solution was extracted, the ethyl acetate layer was rotary evaporated under reduced pressure, the solvent was removed, and then separated and purified by column chromatography to obtain the target product. The eluent of the column chromatography used was petroleum ether and ethyl acetate, and the volume ratio was 10 : 1; The productive rate of product is 77%.
Embodiment 3
[0063] This example provides a 3-trifluoromethyl-substituted pyrazole derivative and a preparation method thereof.
[0064] Its preparation method is as follows: in an air atmosphere, in a 25 ml reaction flask equipped with a reflux condenser, add 1 mmol of benzaldehyde, 1.2 mmol of p-toluenesulfonyl hydrazide, 6 ml of toluene, 3 mmol of lithium tert-butoxide , 2 mmoles of 2-bromo-3,3,3-trifluoropropene, the reaction system was stirred at 60°C for 1 hour, the heating and stirring were stopped, water was added, the reaction liquid was extracted with ethyl acetate, and the ethyl acetate layer was reduced Press rotary steaming, remove the solvent, and then separate and purify by column chromatography to obtain the target product. The column chromatography eluent used is petroleum ether and ethyl acetate, and the volume ratio is 10:1; the yield of the product is 24%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com