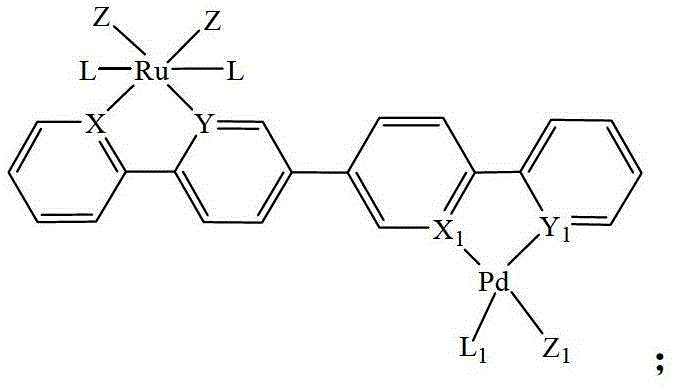
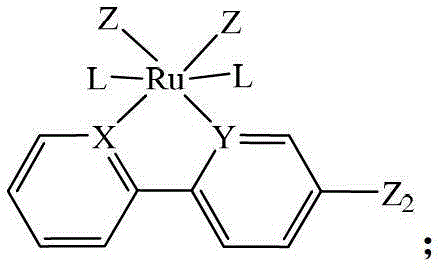
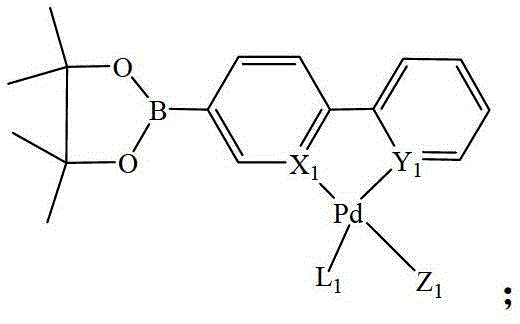
Heteronuclear ruthenium palladium bicyclic metal compound and preparation method and application thereof
A technology of ring metals and compounds, applied in chemical instruments and methods, condensation preparation of carbonyl compounds, organic compounds/hydrides/coordination complex catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation method of the heteronuclear ruthenium palladium bicyclic metal compound in this example is as follows: in a 50ml three-neck flask equipped with a stirring reflux device, add a triethylphosphine mononuclear ruthenium compound (1 mmol) containing a bromine atom, and boronic acid containing Triphenylphosphine mononuclear cyclopalladium compound (1.05mmol) of ester group, potassium carbonate (2.0mmol) and 20ml of anhydrous toluene, stirred and reacted for 12 hours at a temperature of 110°C under a nitrogen atmosphere, filtered, and evaporated the solvent Recrystallized with a mixed solvent of CH2Cl2 and petroleum ether to obtain a yellow product 1 heteronuclear triethylphosphinerutheniumtriphenylphosphinepalladium bicyclic metal compound with a yield of 85.8%. Gained product is carried out nuclear magnetic resonance analysis, data is as follows: 1 H NMR: δ=8.60(d,1H,Ph-H),8.58(d,1H,Ph-H),8.02(d,2H,Ph-H),7.80-7.71(m,14H,Ph-H) ,7.38-7.22(m,9H,Ph-H),6.97(m,2H,...
Embodiment 3
[0056] The preparation method of the heteronuclear ruthenium-palladium bicyclic metal compound in this example is: add bromine-containing triphenylphosphine mononuclear ruthenium compound (1 mmol), boronic acid-containing Triphenylphosphine mononuclear cyclopalladium compound (1.1mmol) of ester group, cesium carbonate (2.5mmol) and 20ml of anhydrous dioxane, stirred and reacted for 10 hours at a temperature of 110°C under a nitrogen atmosphere, filtered, evaporated After removing the solvent, dichloromethane (CH 2 Cl 2 ) and petroleum ether mixed solvent recrystallization to obtain the yellow product 3 heteronuclear triphenylphosphine ruthenium palladium bicyclic metal compound with a yield of 85.2%. Gained product is carried out nuclear magnetic resonance analysis, data is as follows: 1 H NMR: δ=8.63(d,1H,Ph-H),8.53(d,1H,Ph-H),7.98(d,2H,Ph-H),7.85-7.78(m,18H,Ph-H) , 7.38-7.21 (m, 35H, Ph-H), 6.93 (m, 2H, Ph-H).
Embodiment 5
[0058] The preparation method of the heteronuclear ruthenium-palladium bicyclic metal compound in this example is: add bromine-containing triphenylphosphine mononuclear ruthenium compound (1 mmol), boronic acid-containing Trimethylphosphine mononuclear cyclopalladium compound (1.2mmol) of ester group, sodium carbonate (2.5mmol) and 20ml of anhydrous tetrahydrofuran, stirred and reacted for 18 hours at a temperature of 80°C under a nitrogen atmosphere, filtered, and evaporated the solvent with dichloromethane (CH 2 Cl 2 ) and petroleum ether mixed solvent recrystallization to obtain the yellow product 5 heteronuclear triphenylphosphine ruthenium trimethylphosphine palladium bicyclic metal compound with a yield of 92.3%. Gained product is carried out nuclear magnetic resonance analysis, data is as follows: 1 H NMR: δ=8.61(d,1H,Ph-H),8.56(d,1H,Ph-H),8.02(d,2H,Ph-H),7.81-7.75(m,18H,Ph-H) ,7.37-7.28(m,20H,Ph-H),6.95(m,2H,Ph-H),1.08(s,9H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com