Synthesis method of water-soluble fluorescent probe and application of the water-soluble fluorescent probe to detection of antibiotics
A fluorescent probe and synthesis method technology, applied in the field of detection of antibiotics, water-soluble fluorescent probes and their synthesis, can solve the problems of complex detection steps, long detection time, poor practicability, etc., and achieve short detection time, high sensitivity, and responsiveness The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
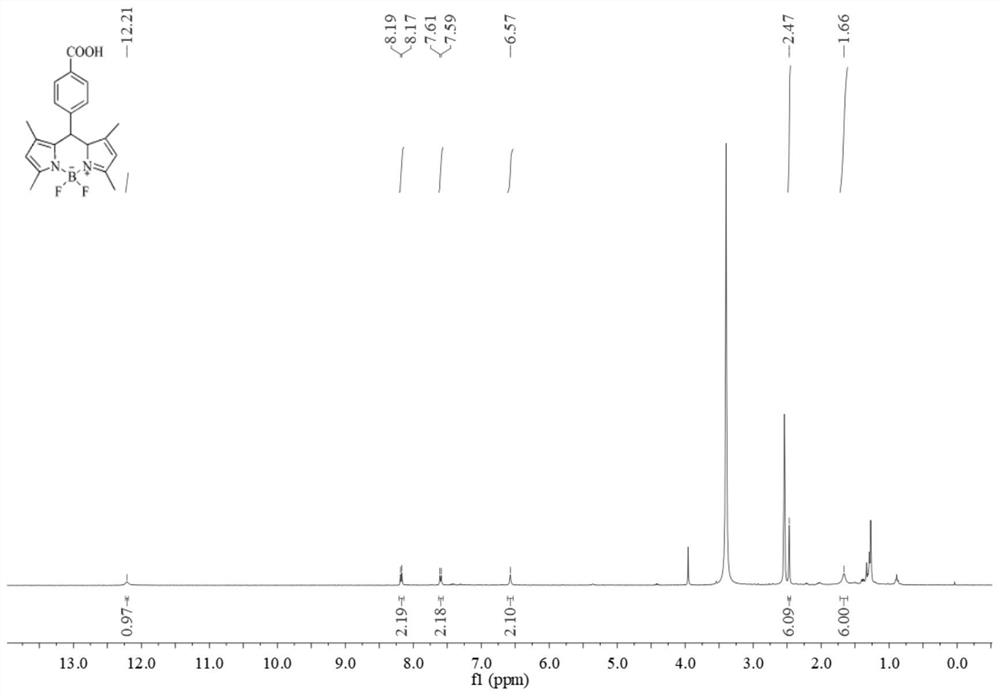
[0037] (1) Under the protection of nitrogen environment, take 410 μL (4 mmol) of 2.4-dimethylpyrrole and 328 mg (2 mmol) of methyl p-formylbenzoate and dissolve them in an appropriate amount of dichloromethane, add 400 μL of trifluoroacetic acid, and stir thoroughly at room temperature for 12 hours , to obtain a mixture.
[0038] (2) Add 454 mg (2 mmol) of DDQ to the mixture in step (1), and stir for 3-5 hours. Add 5ml (4mmol) of triethylamine and fully react for 20min. Add 5ml (4mmol) of boron trifluoride etherate complex and react for 12h to stop the reaction.
[0039] (3) The reaction solution obtained above was washed with saturated brine, extracted three times with dichloromethane, and the organic phase yellow-green solution was collected;
[0040] (4) The yellow-green solution was evaporated and concentrated, separated by silica gel column chromatography, and the bright green fluorescent solution was collected and evaporated and concentrated, then acidified to obtain t...
Embodiment 2
[0042] Embodiment 2 (BODIPY detects OTC):
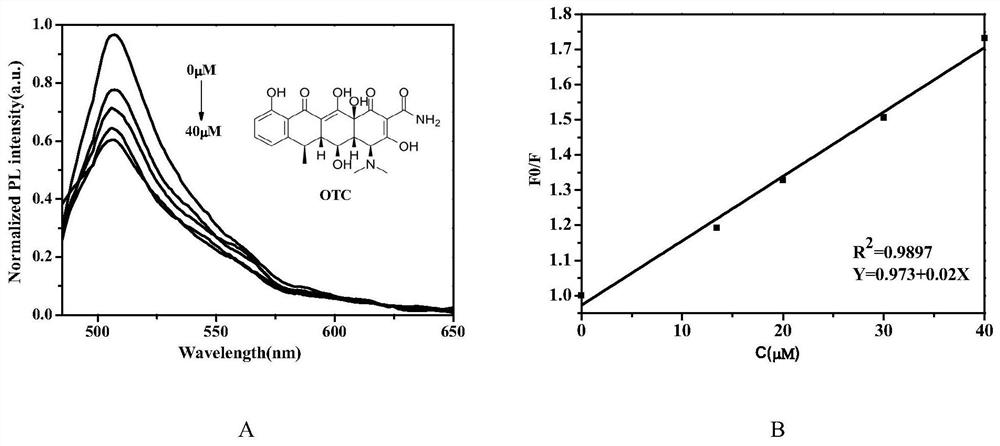
[0043] Concentration gradients of 0μM, 10μM, 20μM, 30μM, 40μM oxytetracycline standard solutions were prepared. Add 33 μM of phosphate buffer solution and blank fluorescent probe sample to 5ml quartz fluorescence cuvette, add equal amount of oxytetracycline standard solution to it in turn, test the fluorescence intensity of blank probe and after adding oxytetracycline each time, as figure 2 (A) shown.
[0044] The fluorescence intensity of the blank fluorescent probe is F0, the fluorescence intensity after adding oxytetracycline sample is F, and the intensity ratio is F0 / F, and the different oxytetracycline concentrations C and the corresponding fluorescence ratio are plotted to draw a standard working curve, such as figure 2 (B) shown. The established linear formula is as follows: Y=0.973+0.02X(R 2 =0.9897), where Y is the ratio of the fluorescence intensity of the blank fluorescent probe to the fluorescence intensity after add...
Embodiment 3
[0048] Embodiment 3 (BODIPY detects TET):
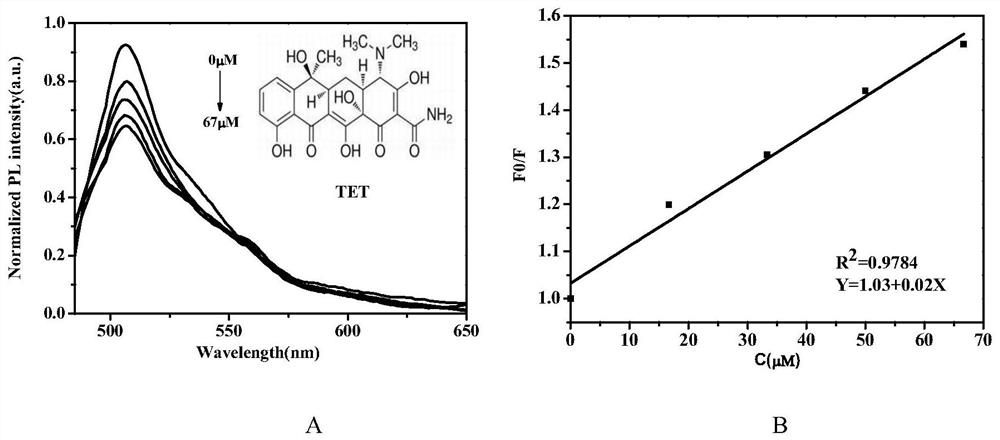
[0049]Prepare tetracycline standard solutions with concentration gradients of 0 μM, 16.67 μM, 33.33 μM, 50 μM, and 66.67 μM. Add phosphate buffer solution and blank fluorescent probe sample 33 μ M to 5ml quartz fluorescent cuvette, add the same amount of tetracycline standard solution to it successively, test the fluorescent intensity of blank probe and adding tetracycline each time, as image 3 (A) shown.
[0050] The fluorescence intensity of the blank fluorescent probe is F0, the fluorescence intensity after adding the tetracycline sample is F, and the intensity ratio is F0 / F, and different tetracycline concentrations C are plotted against the corresponding fluorescence ratio to draw a standard working curve, such as image 3 (B) shown. The established linear formula is as follows: Y=1.03+0.02X(R 2 =0.9784), where Y is the ratio of the fluorescence intensity of the blank fluorescent probe to the fluorescence intensity after add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com