New synthesis method of methyl fluoroacetate and ethyl fluoroacetate
A technology of ethyl fluoroacetate and methyl fluoroacetate, which is applied in the field of preparation of ethyl fluoroacetate, can solve the problems of low efficiency of ethyl fluoroacetate, low utilization rate of equipment, and low fluorinated reagents, and achieve simple post-processing procedures , High fluorination conversion rate, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
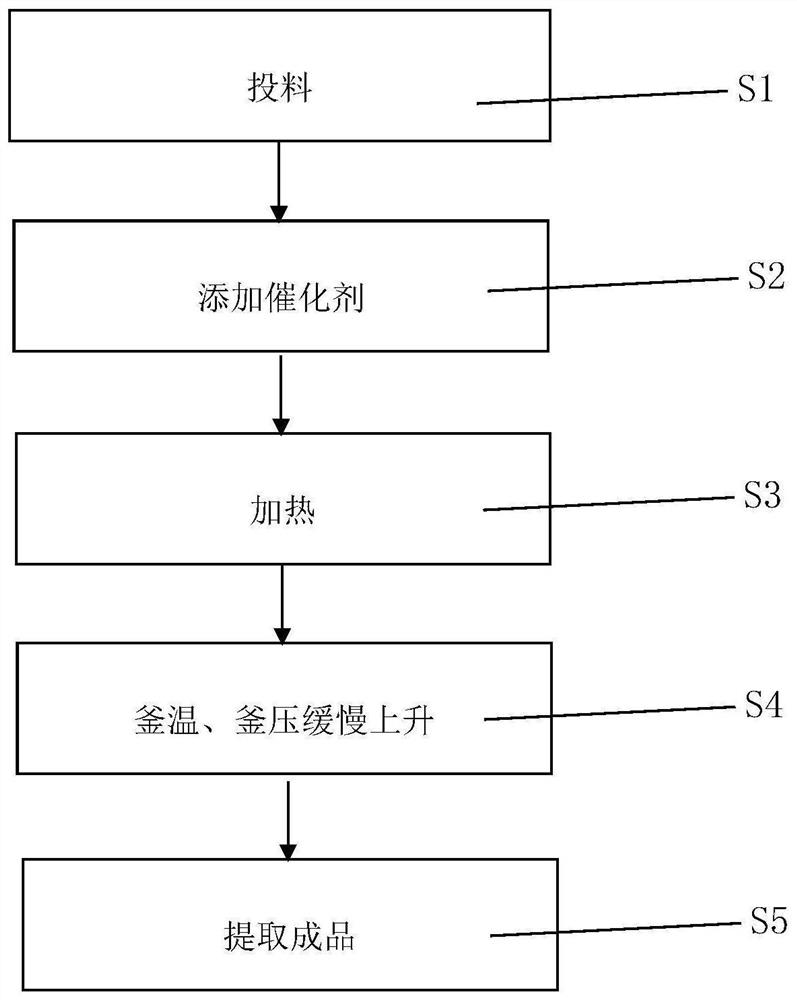
[0024] Such as figure 1 As shown, a new synthetic method of methyl fluoroacetate and ethyl fluoroacetate, the specific steps of the synthetic method are: feeding materials, putting methyl chloroacetate or ethyl chloroacetate into the reaction kettle, opening and stirring; adding catalyst, After stirring evenly, put in potassium fluoride, catalyst A, and catalyst B in turn, and close the feeding hole; heat, use heat transfer oil to heat up to 120°C, and start the reaction. After 1 hour, when the kettle temperature reaches 180°C and the kettle pressure reaches 0.8MPa , turn off the heat conduction oil heating, and start to keep warm; the temperature of the kettle and the pressure of the kettle rise slowly, and continue to keep warm for 3 hours. The material is press-filtered and rinsed, and the filtrate is rectified by a rectification tower to recover chloroacetate to obtain the product methyl fluoroacetate or ethyl fluoroacetate. The product quality can reach more than 99.5%. T...
Embodiment 2
[0031] Such as figure 1 As shown, a new synthetic method of methyl fluoroacetate and ethyl fluoroacetate, the specific steps of the synthetic method are: feeding materials, putting methyl chloroacetate or ethyl chloroacetate into the reaction kettle, opening and stirring; adding catalyst, After stirring evenly, put in potassium fluoride, catalyst A, and catalyst B in turn, and close the feeding hole; heat, use heat transfer oil to heat up to 120°C, and start the reaction. After 1 hour, when the kettle temperature reaches 180°C and the kettle pressure reaches 0.8MPa , turn off the heat conduction oil heating, and start to keep warm; the temperature of the kettle and the pressure of the kettle rise slowly, and continue to keep warm for 3 hours. The material is press-filtered and rinsed, and the filtrate is rectified by a rectification tower to recover chloroacetate to obtain the product methyl fluoroacetate or ethyl fluoroacetate. The product quality can reach more than 99.5%. T...
Embodiment 3
[0038] Such as figure 1 As shown, a new synthetic method of methyl fluoroacetate and ethyl fluoroacetate, the specific steps of the synthetic method are: feeding materials, putting methyl chloroacetate or ethyl chloroacetate into the reaction kettle, opening and stirring; adding catalyst, After stirring evenly, put in potassium fluoride, catalyst A, and catalyst B in turn, and close the feeding hole; heat, use heat transfer oil to heat up to 120°C, and start the reaction. After 1 hour, when the kettle temperature reaches 180°C and the kettle pressure reaches 0.8MPa , turn off the heat conduction oil heating, and start to keep warm; the temperature of the kettle and the pressure of the kettle rise slowly, and continue to keep warm for 3 hours. The material is press-filtered and rinsed, and the filtrate is rectified by a rectification tower to recover chloroacetate to obtain the product methyl fluoroacetate or ethyl fluoroacetate. The product quality can reach more than 99.5%. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com