Oxidation of hydrocarbons to acids in the presence of fluoro compounds
A technology of fluorine compounds and mixtures, applied in the field of dibasic acids, can solve the problem of unnoticed importance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
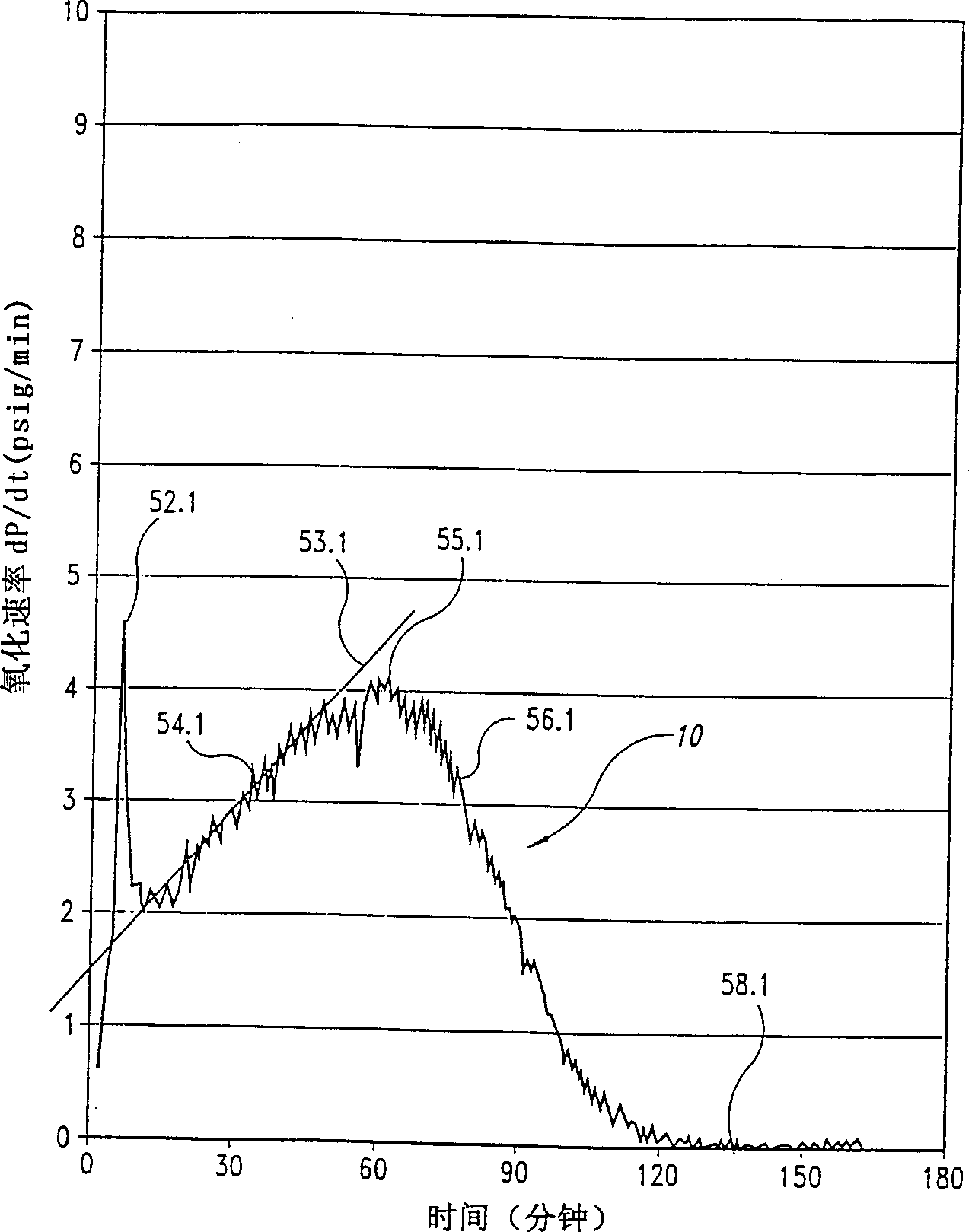
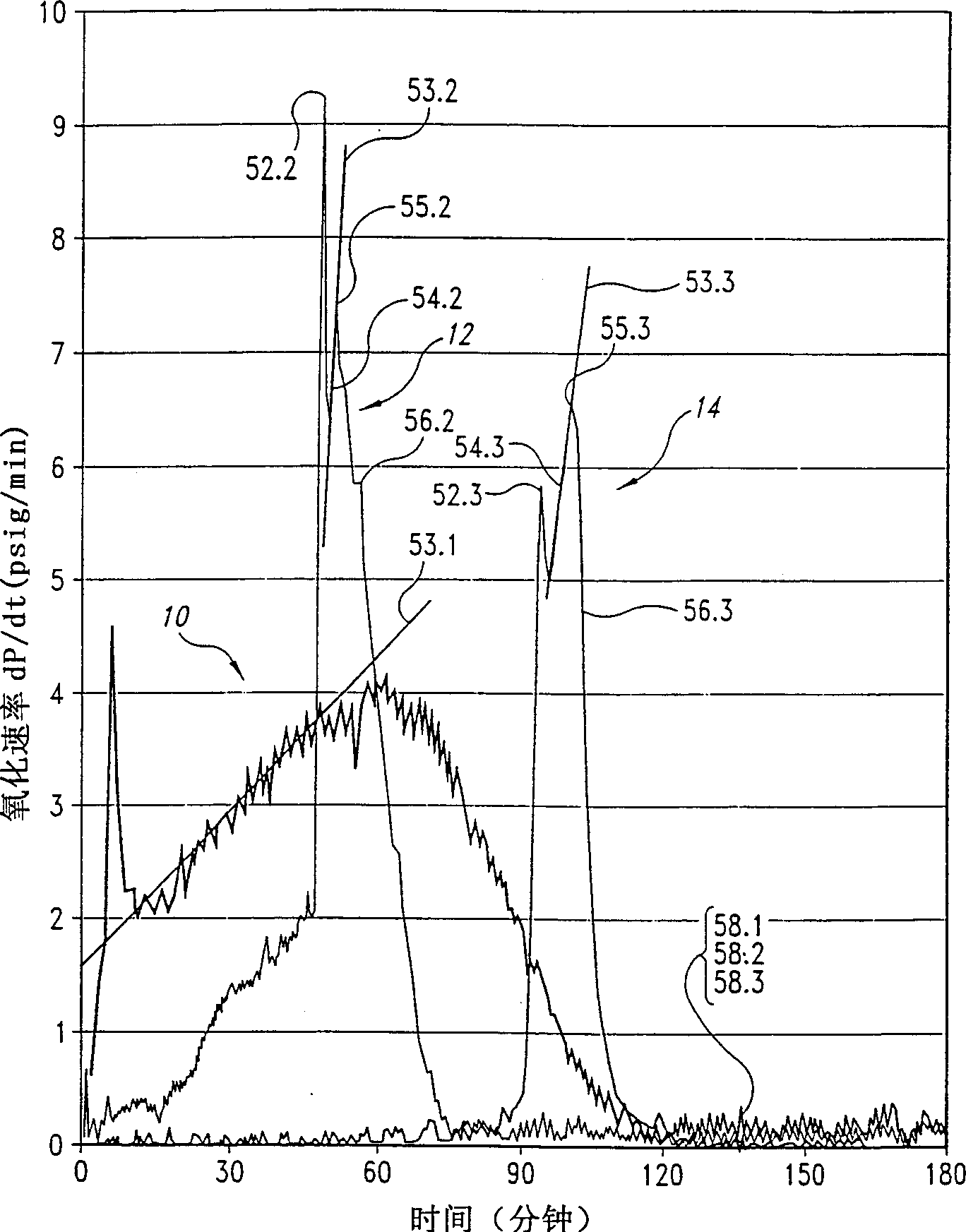
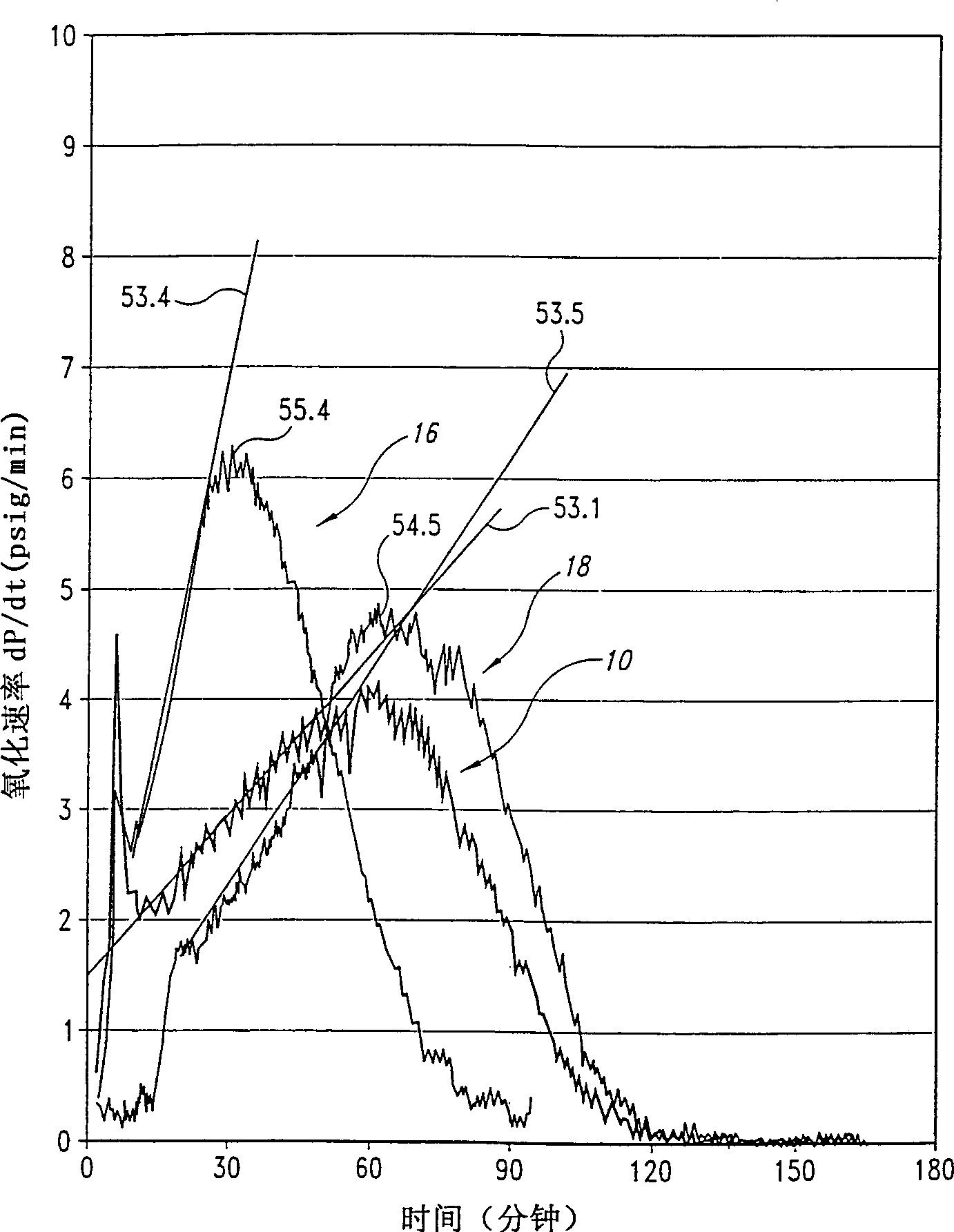
[0045] As stated above, the present invention relates to a process for the preparation of dibasic acids by oxidizing a hydrocarbon with a gas containing an oxidizing agent, preferably oxygen.
[0046] The inventors found that during the oxidation of hydrocarbons such as cyclohexane and xylene to form the corresponding dibasic acids in the presence of a cobalt catalyst, adding a critical amount of fluorine compounds to the reaction mixture can increase the reaction rate without compromising selectivity, yield and / or response control.
[0047] Despite the practical importance of continuous systems for the production of dibasic acids compared to batch reactors, the inventors have found that microreactors can be used to determine the limits within which continuous systems operating under steady conditions can be advantageously utilized Fluorine compounds.
[0048] Two critical parameters relate to the aforementioned limits and were determined with the microreactor described below...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com