Method for determining content and associated substances of sorafenib tosylate in high-performance liquid phase chromatography
A technology of high performance liquid chromatography and toluenesulfonic acid, applied in the field of pharmaceutical analysis, can solve the problems of inability to achieve separation and effective control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
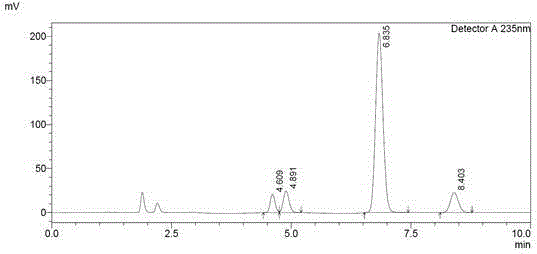
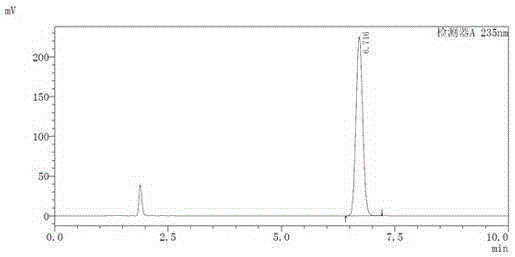
[0027] The specific operation steps of HPLC assay Sorafenib tosylate content are as follows:
[0028] (1) Select 14mg of sorafenib tosylate, weigh it accurately, place it in a 100ml measuring bottle, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution;
[0029] (2) Take another 14 mg of sorafenib tosylate, and prepare it so that the concentration of sorafenib is 0.1 mg / ml, as a control solution;
[0030] (3) Take 10 μl of the test solution and the control solution respectively, inject them into the chromatograph, record the chromatogram, calculate the peak area according to the external standard method and fold it to dryness.
Embodiment 2
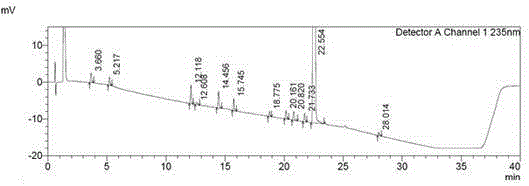
[0032] The specific operation steps of high-performance liquid chromatography determination sorafenib tosylate related substances are as follows:
[0033] (4) Take 14mg of sorafenib tosylate, accurately weigh it, place it in a 10ml measuring bottle, add a mixed solvent, the mixed solvent is a mixture of water, acetonitrile and ethanol, and the mixture of water, acetonitrile and ethanol The volume ratio is 250:450:300, by ultrasound, sorafenib tosylate is fully dissolved, shaken up, as the test solution;
[0034] (5) Accurately measure 1ml of the test solution, place it in a 100ml measuring bottle, dilute to the mark with the mixed solvent in step (4), and shake well; then precisely measure 1ml of the solution obtained in the first stage of step (5) , placed in a 10ml measuring bottle, dilute to the mark with the mixed solvent in step (4), shake well, and use it as a control solution;
[0035] (6) Take 10 μl of the control solution and inject it into the liquid chromatograph, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com