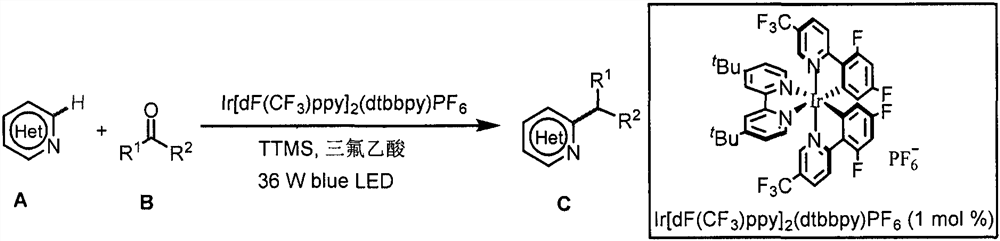
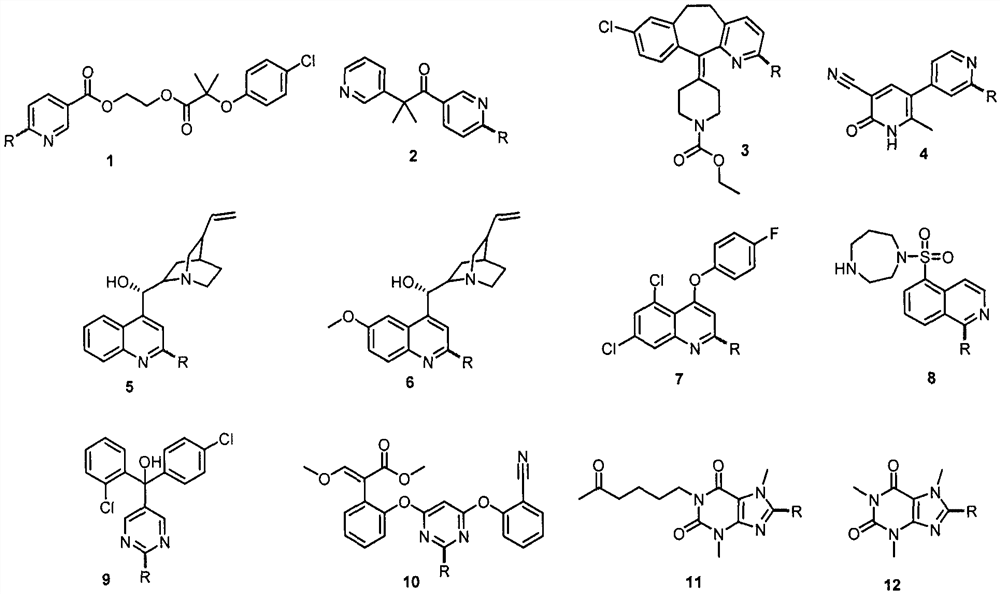
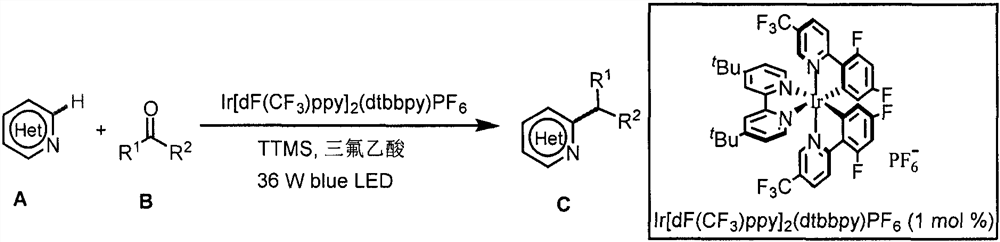
Application of light-promoted Minissci C-H alkylation reaction in preparation of alkyl-substituted azacycle
A technology of miniscic-h and alkylation reaction, applied in the field of fine chemicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Synthesis of 2-(2-(4-chlorophenoxy)-2-methylpropionyl)oxy)ethyl 6-isopropylnicotinate:
[0033] Weigh 0.3mmol 2-((2-(4-chlorophenoxy)-2-methylpropionyl)oxy) ethyl nicotinate, 0.003mmol photocatalyst [Ir(dF(CF 3 )ppy) 2 (dtbbpy)]PF 6 , 0.6mmol tris-(trimethylsilyl) silane (TTMS), 0.6mmol trifluoroacetic acid (TFA) in 8mL reaction flask, then add 3mL acetone solution, blow argon gas into the reaction flask for 30s, at 490nm at 36W Reaction under blue light irradiation, TLC monitoring until the raw material reaction is complete, then add 30mL sodium bicarbonate solution, 30mL dichloromethane solution, liquid separation, the organic layer is dried with anhydrous sodium sulfate, spin off the solvent, column chromatography (petroleum ether: acetic acid ethyl ester=4:1) to obtain a colorless liquid with a yield of 38%. 1 H NMR (400MHz, CDCl 3)δ9.07(d, J=1.6Hz, 1H), 8.04(dd, J=8.0, 2.0Hz, 1H), 7.23(d, J=8.0Hz, 1H), 7.10(d, J=8.8Hz, 2H), 6.76(d, J=8.8Hz, 2H), 4.5...
Embodiment 2
[0034] Example 2: Synthesis of 2-(2-(4-chlorophenoxy)-2-methylpropionyl)oxy)ethyl 6-cyclohexylnicotinate:
[0035] Weigh 0.3mmol 2-((2-(4-chlorophenoxy)-2-methylpropionyl)oxy) ethyl nicotinate, 0.003mmol photocatalyst [Ir(dF(CF 3 )ppy) 2 (dtbbpy)]PF 6 , 0.6mmol tris-(trimethylsilyl)silane (TTMS), 0.6mmol trifluoroacetic acid (TFA) in 8mL reaction flask, 9mmol cyclohexanone, then add 1.5mL acetonitrile solution, blow argon into the reaction flask 30s, react under 36W 490nm blue light irradiation, TLC monitors until the raw materials have reacted completely, then add 30mL sodium bicarbonate solution, 30mL dichloromethane solution, separate the liquid, dry the organic layer with anhydrous sodium sulfate, spin off the solvent, and the column layer Analysis (petroleum ether: ethyl acetate = 4:1) gave a yellow liquid with a yield of 50%. 1 H NMR (400MHz, CDCl 3 )δ9.06(s, 1H), 8.04(d, J=8.0Hz, 1H), 7.22(d, J=8.0Hz, 1H), 7.09(d, J=8.0Hz, 2H), 6.76(d, J=8.0Hz, 2H), 4.53(s, 4H), 2....
Embodiment 3
[0036] Example 3: Synthesis of 1-(6-isopropylpyridin-3-yl)-2-methyl-2-(pyridin-3-yl)propan-1-one:
[0037] Weigh 0.3mmol 2-methyl-1,2-bis(pyridin-3-yl)propan-1-one, 0.003mmol photocatalyst [Ir(dF(CF 3 )ppy) 2 (dtbbpy)]PF 6 , 0.6mmol tris-(trimethylsilyl) silane (TTMS), 0.6mmol trifluoroacetic acid (TFA) in 8mL reaction flask, then add 3mL acetone solution, blow argon gas into the reaction flask for 30s, at 490nm at 36W Reaction under blue light irradiation, TLC monitoring until the raw material reaction is complete, then add 30mL sodium bicarbonate solution, 30mL dichloromethane solution, liquid separation, the organic layer is dried with anhydrous sodium sulfate, spin off the solvent, column chromatography (dichloromethane: Methanol=20:1) to obtain a white solid with a yield of 35%. Melting point 60-61℃. 1 H NMR (400MHz, CDCl 3 )δ8.58(d, J=20.0Hz, 3H), 7.80(d, J=7.2Hz, 1H), 7.61(d, J=8.0Hz, 1H), 7.30(dd, J=7.6, 5.2Hz, 1H), 7.11(d, J=8.4Hz, 1H), 3.11-2.95(m, 1H), 1.66(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com