Energetic crystal material obtained through self-assembly of melamine nitrogen oxide and oxidant and preparing method thereof
A technology of melamine nitrogen oxide and polycyanamide nitrogen oxide, which is applied in the field of energetic crystal materials and its preparation, can solve the problems of high cost of raw materials, difficulty in effectively reconciling energy and sensitivity, complex preparation process, etc., and achieve simple preparation process , Low raw material cost, high detonation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
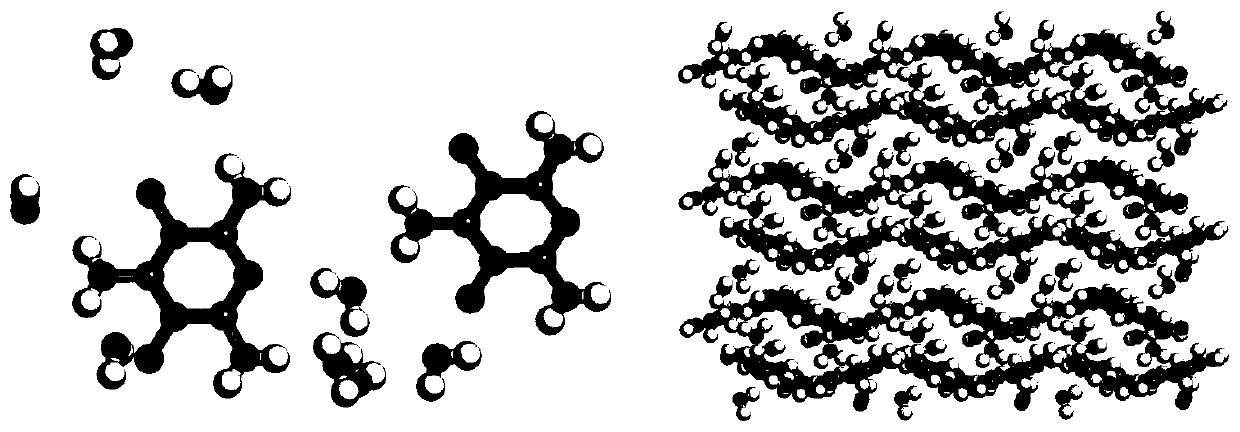
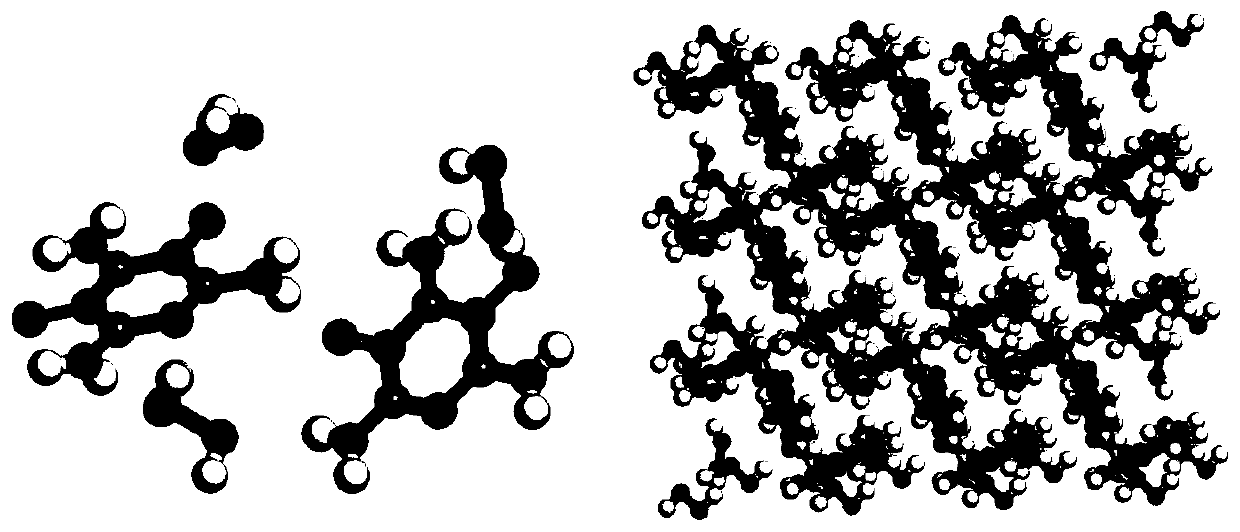
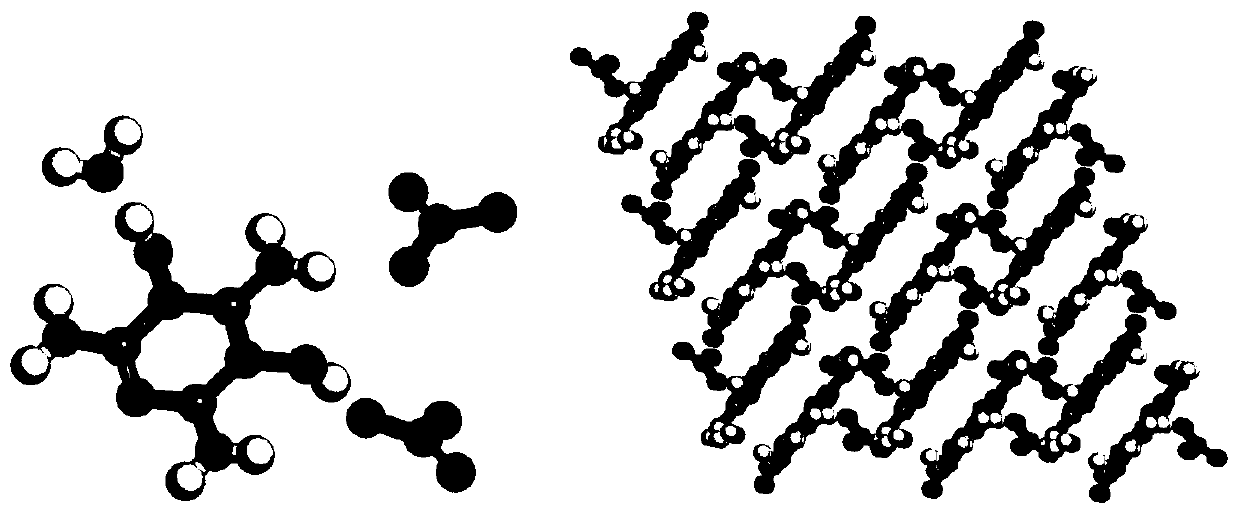
[0041] At 30°C, add trifluoroacetic acid into the three-necked flask, then pour in a sufficient amount of melamine under stirring, dissolve, and then slowly add a sufficient amount of 30% hydrogen peroxide dropwise. After reacting for 6 hours, a white precipitate was obtained by filtration. Dissolve the white precipitate in an appropriate amount of water, add sodium bicarbonate to neutralize to a pH of 7, filter to obtain melamine nitrogen oxide, and the crystal structure of melamine nitrogen oxide hydrate is shown in the attached figure 1 shown.
Embodiment 2
[0043] At 60°C, add trifluoroacetic acid into the three-necked flask, then pour in a sufficient amount of melamine under stirring, dissolve, and then slowly add a sufficient amount of 30% hydrogen peroxide dropwise. After reacting for 24 hours, a white precipitate was obtained by filtration. Dissolve the white precipitate in an appropriate amount of water, add sodium hydroxide to neutralize to a pH of 7, and filter to obtain melamine nitrogen oxide.
Embodiment 3
[0045] At 50°C, add hydrochloric acid into the three-necked flask, then add enough melamine under stirring to dissolve, and then slowly add a sufficient amount of 30% hydrogen peroxide dropwise. After reacting for 6 hours, a white precipitate was obtained by filtration. Dissolve the white precipitate in an appropriate amount of water, add sodium carbonate to neutralize to a pH of 7, and filter to obtain melamine nitrogen oxide. The reaction scheme is as follows:
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com