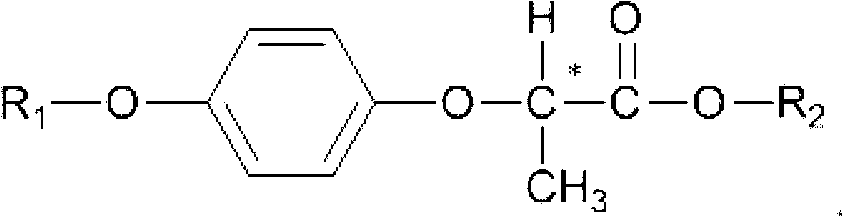
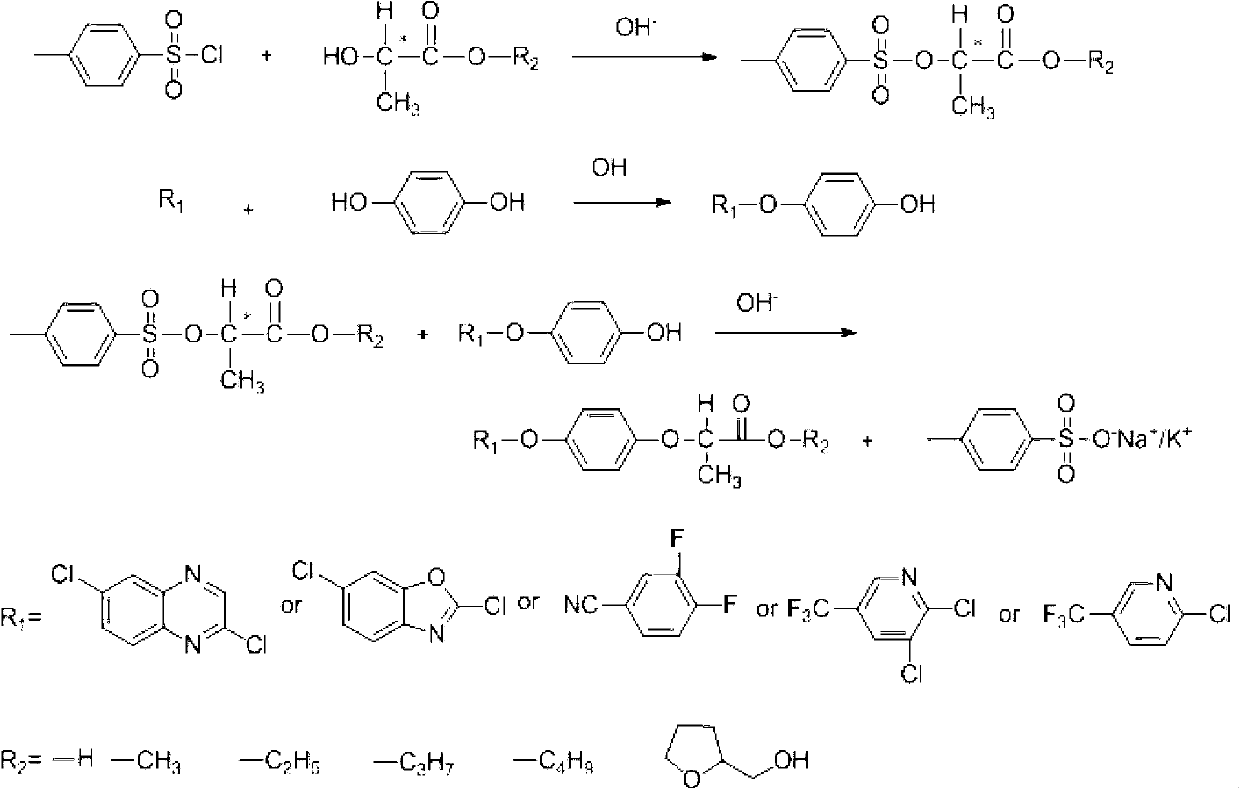
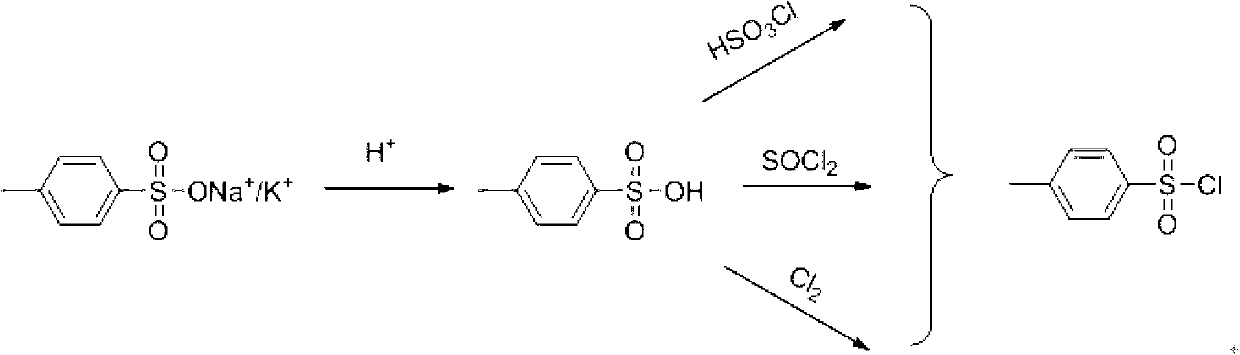
Method for recovering paratoluensulfonyl chloride from waste water generated by producing aryloxy phenoxy propionic acid herbicide
A technology of aryloxyphenoxypropionic acid and p-toluenesulfonyl chloride is applied in the field of organic chemical wastewater treatment and recovery, and achieves the effects of simple process flow, reduction of COD content, and large process production effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Taking quizalofop-p-ethyl production wastewater as an example, p-toluenesulfonyl chloride was prepared with chlorine gas as the acid chloride reagent and dimethylformamide (DMF) as the polar solvent.
[0037] Take 200g of waste water containing p-toluenesulfonate from the workshop and put it into a 500ml four-necked bottle, add 19.0g (0.187mol) concentrated hydrochloric acid at room temperature to acidify to pH 1-2, after acidification, add 4.0g of activated carbon to decolorize for 1.0h, Suction filtration, the filtrate was distilled at normal pressure, after 100g of water was distilled off, 100g of toluene was added to the system to carry out normal pressure reflux with water until the system was free of water. 38 g (0.17 mol) of solid were obtained by suction filtration.
[0038] Put the obtained solid into a 250ml four-necked bottle, add 76g of dichloromethane and 2.50g (0.034mol) of DMF at the same time, start to feed chlorine gas into the system for 6-7...
Embodiment 2
[0039] Example 2: Taking quizalofop-p-ethyl production wastewater as an example, p-toluenesulfonyl chloride was prepared using thionyl chloride as the acid chloride reagent and DMF as the reaction solvent.
[0040] Take 200g of waste water containing p-toluenesulfonate from the workshop into a 500ml four-neck bottle, add 19.5g (0.188mol) concentrated hydrochloric acid at room temperature to acidify to pH 1-2, after acidification, add 4.0g of activated carbon for decolorization for 1.0h, Suction filtration, the filtrate was distilled at normal pressure, after 100g of water was distilled off, 100g of toluene was added to the system to carry out normal pressure reflux with water until the system was free of water. Suction filtration yielded 38.2 g (0.171 mol) of solid.
[0041] Put the obtained solid into a 250ml four-necked bottle, add 76g of dichloromethane and 2.50g (0.034mol) of DMF at the same time, add 24.49g (0.204mol) of thionyl chloride dropwise at 20-25 degrees, and the...
Embodiment 3
[0042] Example 3: Taking quizalofop-p-ethyl production wastewater as an example, p-toluenesulfonyl chloride was prepared using chlorosulfonic acid as the acid chloride reagent.
[0043] Take 200g of waste water containing p-toluenesulfonate from the workshop into a 500ml four-necked bottle, add 18.7g (0.186mol) concentrated hydrochloric acid at room temperature to acidify to pH 1-2, after acidification, add 4.0g of activated carbon for decolorization for 1.0h, Suction filtration, the filtrate was distilled at normal pressure, after 100g of water was distilled off, 100g of toluene was added to the system to carry out normal pressure reflux with water until the system was free of water. Suction filtration yielded 38.2 g (0.171 mol) of solid.
[0044] Put the obtained solid into a 250ml four-neck bottle, add 76.4g of dichloroethane at the same time, add 40.80g (0.34mol) of chlorosulfonic acid dropwise at 40-70 degrees, and complete the reaction in 1h, add 100g of ice water to was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com