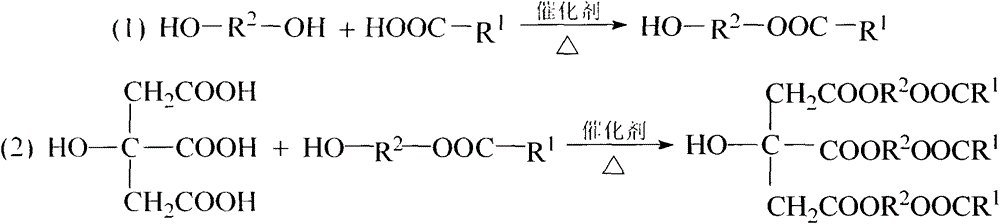
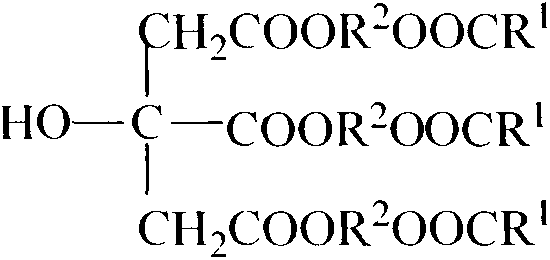Synthesis of citric acid ether ester plasticizer
A citric acid ester and plasticizer technology, which is applied in the field of synthesis and preparation of new green and environmentally friendly citrate plasticizers, can solve the problems of poor durability and reduced compatibility of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: the synthesis of tri(butylene glycol monobutyl ester) citrate
[0015] ①Add 1mol (88.11g) of butyric acid, 1.2mol (108.14g) of butanediol, 0.94g of p-toluenesulfonic acid, and 25ml. Heat to 120°C and reflux for 4 hours from the first drop of water. During the reaction, the water phase in the lower layer of the water separator is continuously released and collected with a measuring cylinder to measure the water output. After the esterification reaction, distill off excess toluene under reduced pressure, then cool the reaction product below 60°C, transfer it into a separatory funnel and add an appropriate amount of distilled water, shake it fully and let it stand for 30 minutes. The distilled water and the organic phase are separated, and the lower water phase is released , the upper organic phase was poured into a flask and distilled and purified at a temperature of 95° C. and a pressure of 0.01 MPa to obtain butanediol monobutyl ester.
[0016] After the...
Embodiment 2
[0019] Embodiment 2: the synthesis of tri(diethylene glycol unit ethyl ester) citrate
[0020] ①Add 1mol (60.05g) of acetic acid, 1.2mol (127.33g) of diethylene glycol, 0.94g of p-toluenesulfonic acid, and 25ml of toluene into a 500ml four-neck flask equipped with a stirrer, water separator, serpentine condenser and thermometer . Heat to 120°C and reflux for 4 hours from the first drop of water. During the reaction, the water phase in the lower layer of the water separator is continuously released and collected with a measuring cylinder to measure the water output. After the esterification reaction, distill off the excess toluene under reduced pressure, then cool the reaction product to below 60%, then transfer it into a separatory funnel and add appropriate amount of distilled water and NaCl, shake it fully and let it stand in a water bath at 80°C for 30min, then separate the distilled water from the organic phase layer, release the organic phase and pour it into a flask at ...
Embodiment 3
[0024] Embodiment 3: the synthesis of tri(triethylene glycol monobutyl ester) citrate
[0025] ①Add 1mol (88.11g) of butyric acid, 1.2mol (180.20g) of triethylene glycol, 1.34g of p-toluenesulfonic acid, and 35ml. Heat to 120°C and reflux for 4 hours from the first drop of water. During the reaction, the water phase in the lower layer of the water separator is continuously released and collected with a measuring cylinder to measure the water output. After the esterification reaction, distill off excess toluene under reduced pressure, then cool the reaction product below 60°C, then transfer it into a separatory funnel and add appropriate amount of distilled water and NaCl, shake it fully and let it stand in a water bath at 80°C for 30 minutes, then separate the distilled water from the organic phase layer, release the organic phase and pour it into a flask to obtain triethylene glycol monobutyl ester after distillation and purification under reduced pressure at 100°C.
[0026...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com