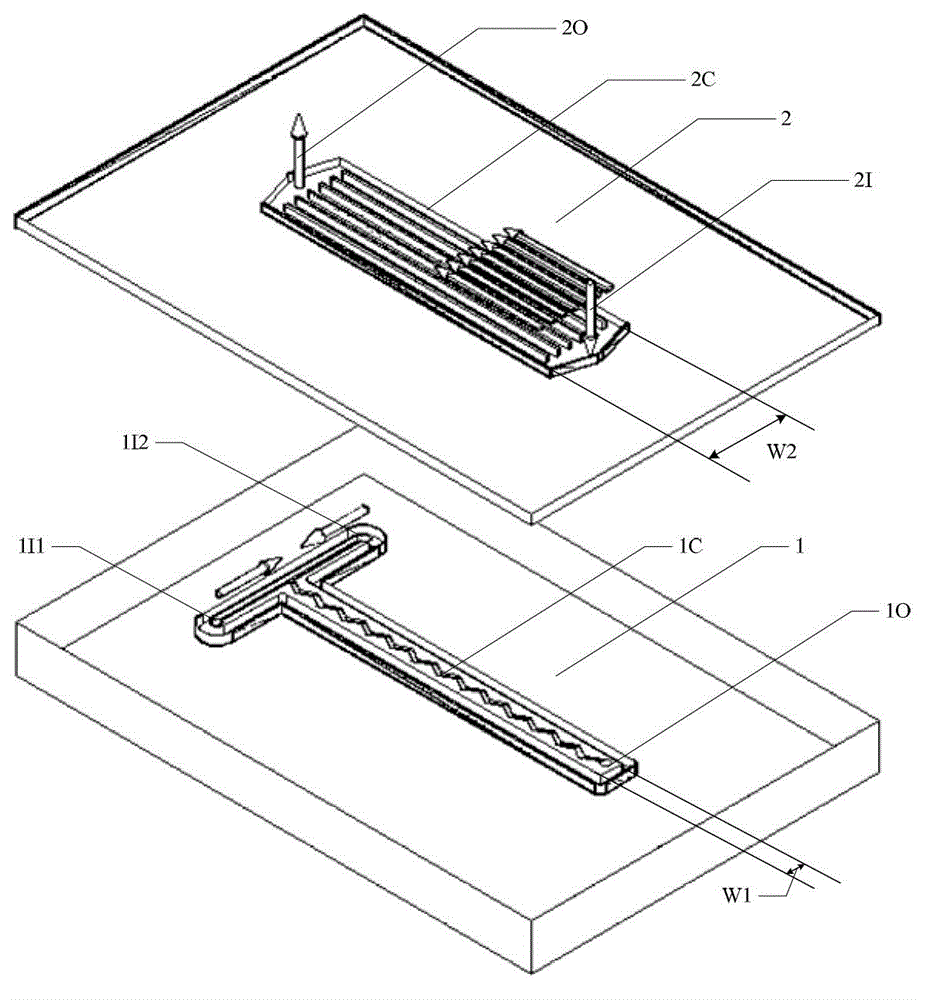
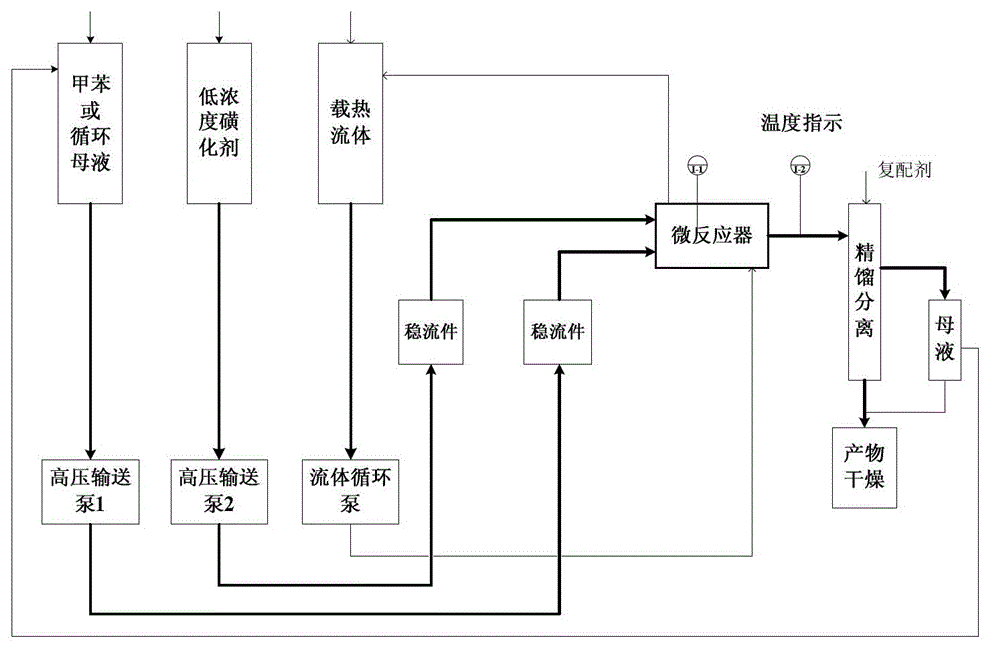
Method for preparing p-toluenesulfonic acid through toluene sulfonation
A technology of p-toluenesulfonic acid and toluene, which is applied in the fields of sulfonic acid preparation, chemical recovery, organic chemistry, etc., can solve the problems of sulfonation microreactor technology without available data, and achieve the effect of safe and continuous process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Low concentration, equimolar reaction process
[0022] Reaction operating conditions: the initial concentration of toluene is 10wt% (solvent is 1,2-dichloroethane), the initial concentration of sulfur trioxide is 5wt% (solvent is 1,2-dichloroethane), adjust the reactant toluene and trichloroethane The flow rate of the sulfur oxide solution is such that the molar ratio of toluene to sulfur trioxide is 1:1, and the ratio of the sum of the total volume flow rate of the two to the volume of the reaction microchannel is 13000 hours -1 (That is, the apparent residence time is 0.28s), and the reaction is performed at a reaction temperature of 28°C. The product is separated by filtration. The separated solid is placed in an oven at 50°C and dried to constant weight. Weigh a certain mass of solid product and dissolve it in triethyl orthoformate, and esterify it at 115°C for 1 hour. The esterified product is analyzed by gas chromatography. Gas chromatographic conditions: ...
Embodiment 2
[0024] Example 2: Isometric, recirculation process
[0025] Referring to Example 1, it can be seen that the toluene concentration is 10wt.%, SO 3 The concentration of 5wt.%, the reaction temperature is close to normal temperature, the content of p-toluenesulfonic acid in the product is nearly 95%. According to this result, the toluene concentration of the reactant is increased to make it excessive. After the reaction, the concentration of toluene in the filtered mother liquor is reduced according to the reaction rate. Reflux continues to react as a reactant without affecting the product concentration. Therefore, the present invention proposes a mother liquor circulation and low-temperature microreaction process.
[0026] The operating conditions are: the initial concentration of toluene is 50wt% (solvent is 1,2-dichloroethane), the initial concentration of sulfur trioxide is 5wt% (solvent is 1,2-dichloroethane), the reaction temperature is 28°C, The residence time is 0.28s. Feed an...
Embodiment 3
[0029] Example 3: High-concentration, equal-volume, recirculation process
[0030] Referring to Example 2, it can be seen that in the toluene concentration of 50wt.%, SO 3 The concentration is 5wt.%, the reaction temperature is close to normal temperature, and the p-toluenesulfonic acid content in the product reaches 96.36% under the operating conditions of the three-cycle reflux process. It is analyzed that the by-product sulfone circulates with the mother liquor as a reaction material, which inhibits the formation of phenylsulfone. Can increase the concentration of p-toluenesulfonic acid product. According to this result and inference, continue to increase the toluene concentration of the reactant to make it excessive and reduce the amount of solvent. After the reaction, the toluene concentration in the filtered mother liquor is gradually reduced according to the reaction rate, and multiple refluxes are used as the reactant to continue the reaction without affecting the product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com