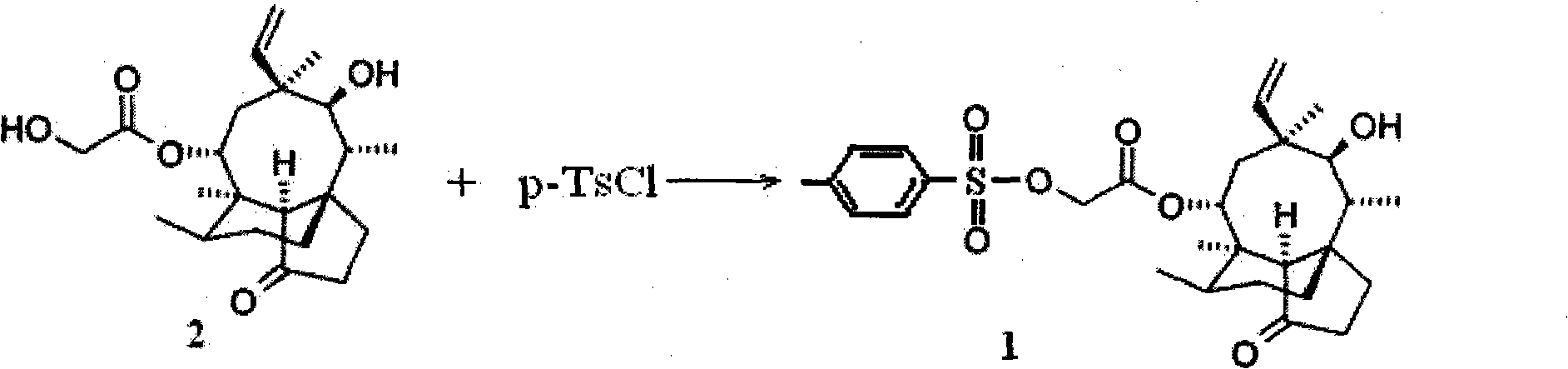
Synthesis method of p-toluene sulfonic acid pleuromutilin ester
A technology of pleuromutilin ester and p-toluenesulfonic acid, which is applied in the preparation of sulfonic acid ester, organic chemistry and other directions, can solve the problems of low production efficiency, long reaction time, low catalytic performance of triethylamine, etc., and achieves improved synthesis efficiency. and productivity, environmentally friendly worker health, and the effect of shortening the synthesis reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 378.5 grams (1mol) of pleuromutilin were added to 1500 milliliters of methyl isobutyl ketone, and then 209.4 grams (1.1mol) of p-toluenesulfonyl chloride and a content of 40 grams (1mol), 30% sodium hydroxide solution in water. After the dropwise addition, the temperature was raised to 60° C. for 0.5 hour reaction. After the reaction solution was cooled to room temperature, the organic layer was separated, washed with water (800 ml × 3), dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 506.6 grams of white pleuromutilin p-toluenesulfonate Solid, yield 95.1%, its chromatographic purity analyzed by high pressure liquid chromatography is 97%.
Embodiment 2
[0017] 378.5 grams (1mol) of pleuromutilin were added to 1500 milliliters of methyl isobutyl ketone, and then 209.4 grams (1.1mol) of p-toluenesulfonyl chloride and 56 grams (1mol) of concentration were added under stirring. It is 25% potassium hydroxide aqueous solution. After the dropwise addition, the temperature was raised to 50° C. and the reaction was carried out for 0.5 hours. After the reaction solution was cooled to room temperature, the organic layer was separated, washed with water (800 ml×3), dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 514.0 grams of white pleuromutilin p-toluenesulfonic acid solid , the yield was 96.5%, and its chromatographic purity was 97.2% as analyzed by high pressure liquid chromatography.
Embodiment 3
[0019] Add 189.3 grams (0.5 mol) of pleuromutilin to 800 ml of methyl isobutyl ketone, then add 104.7 grams (0.55 mol) of p-toluenesulfonyl chloride and a content of 53 grams (0.5 mol) of saturated aqueous sodium carbonate solution. After the dropwise addition, the temperature was raised to 65° C. and the reaction was carried out for 1.5 hours. After the reaction solution was cooled to room temperature, the organic layer was separated, washed with water (600 ml × 3), dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 253.0 grams of white pleuromutilin p-toluenesulfonic acid solid , the yield was 95.0%, and its chromatographic purity was 98.1% as analyzed by high-pressure liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com