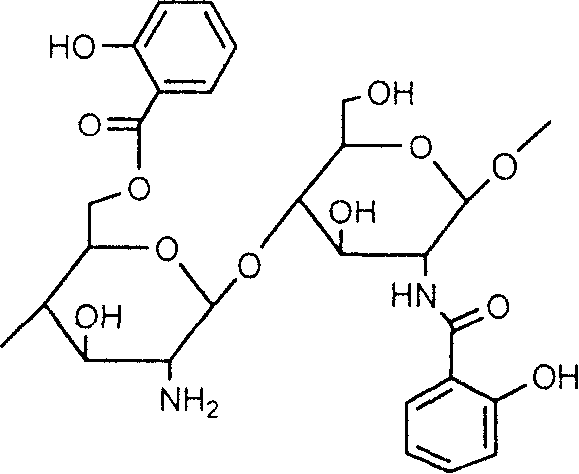
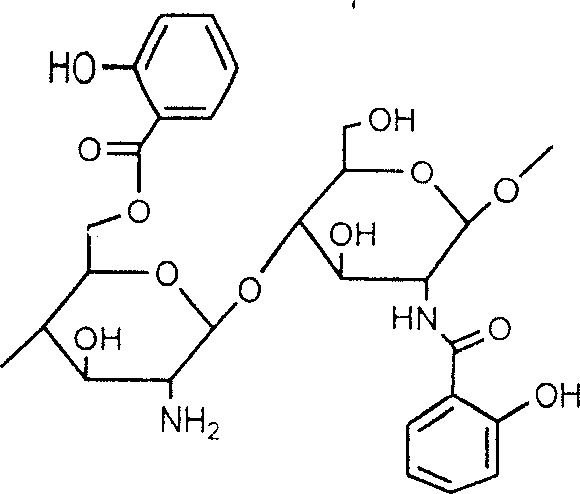
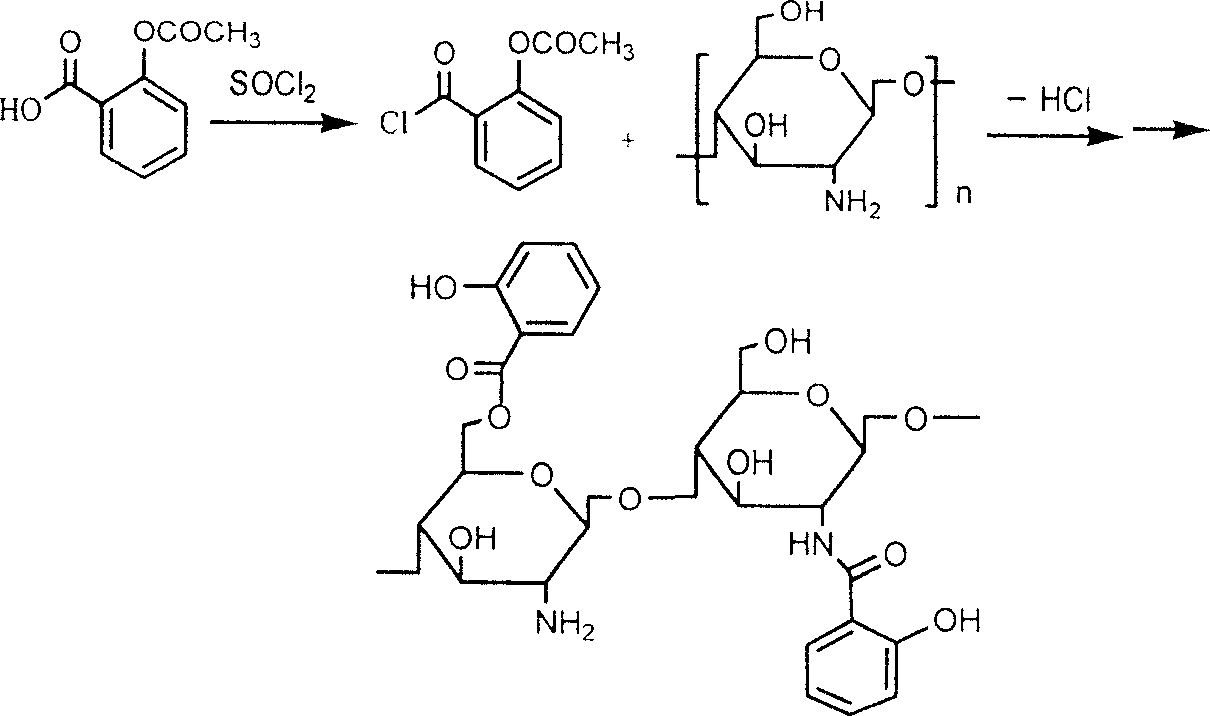
Salicylic acid and chitin-2-6-bit graft and its production
A technology of chitosan and graft, applied in the field of salicylic acid and chitosan-2, can solve the problems of inability to be taken orally, strong irritation of salicylic acid, gastric perforation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1. Add an appropriate amount of acetylsalicylic acid into the reaction flask, slowly dropwise add freshly distilled thionyl chloride (added at a molar ratio of 1:1), and stir under reflux for 3 hours to obtain A solution.
[0024] Swell an appropriate amount of chitosan with methanesulfonic acid, slowly add equimolar (chitosan is calculated as monosaccharide unit) A solution dropwise, stir and react at 20° C. for 2 hours, and let stand. Add a large amount of acetone, separate out the precipitate, neutralize the precipitate with ammonia water, wash with water, wash with ethanol, and spray dry or freeze dry the solid to obtain a derivative mainly grafted at the 6-position, with an average grafting rate of about 60%.
Embodiment 2
[0025] Example 2: Add chitosan / acetylsalicylic acid with a weight-feeding ratio of 6:5 to a reaction flask, add thionyl chloride solution dropwise, and stir under reflux for 3 hours to obtain a solution.
[0026] Put chitosan with chitosan / acetylsalicylic acid weight ratio of 6:5 into 15 times the amount of dioxane or DMF, add p-toluenesulfonic acid to swell, and react with solution A at 20°C 2hr, stand still. Add a large amount of ammonia to neutralize, let stand, and centrifuge. The precipitate is washed with water and ethanol, and the solid is spray-dried or freeze-dried to obtain a derivative mainly grafted at the 6-position, with an average grafting rate of about 50%.
Embodiment 3
[0027] Example 3: Add chitosan / acetylsalicylic acid with a weight-feeding ratio of 6:5 to a reaction flask, add thionyl chloride solution dropwise, and stir under reflux for 3 hours to obtain A solution.
[0028] Add chitosan with chitosan / acetylsalicylic acid weight ratio of 6:5 into the reaction flask, use dimethyl sulfoxide or DMF as solvent, and add a little pyridine or triethylamine. Slowly drop the A solution into the reaction bottle, keep the temperature at 20°C, stir for 3 hours, and let stand. Filter, wash the product with ethanol, and centrifuge to obtain 2-position and 6-position mixed graft derivatives, with an average grafting rate of about 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com