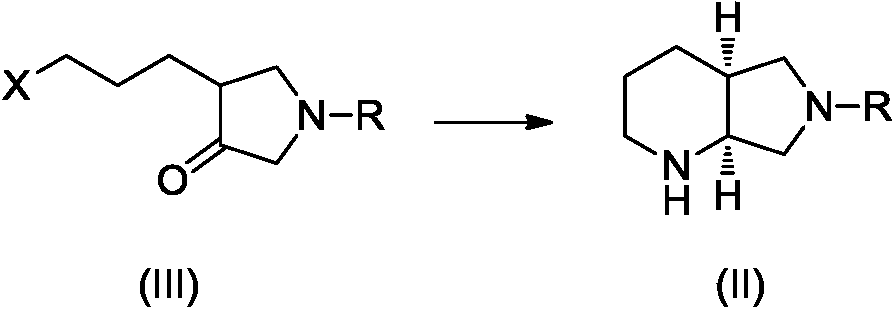
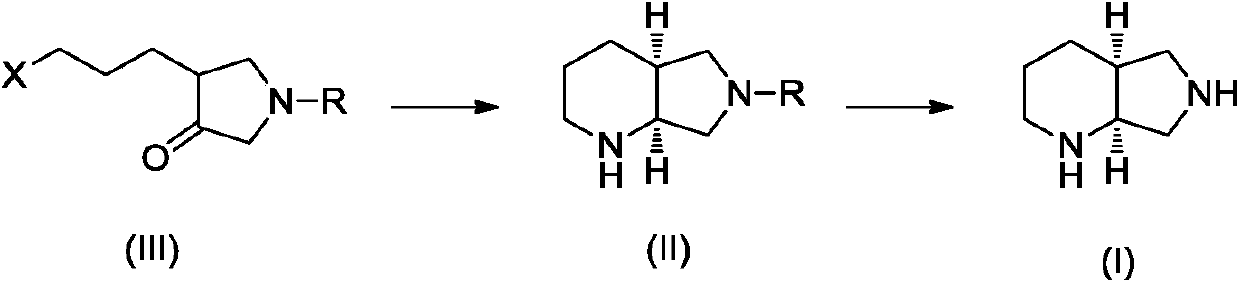
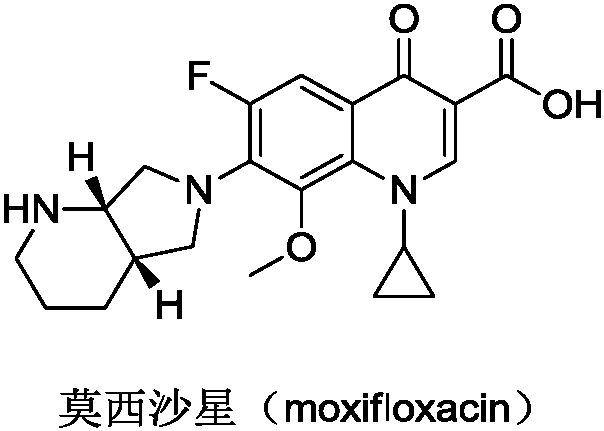
Preparation method of moxifloxacin intermediate compound
A compound and donor technology, applied in the field of preparation of moxifloxacin intermediate compounds, can solve the problems of low product ee value, complicated process and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1. Transamination reaction
[0062]
[0063] Add 15.0 g of ethyl 3-(3-bromopropyl)-4-oxopyrrolidine-1-carboxylate (3-1) into 400 mL of distilled water, add 20 mL of DMSO to aid dissolution, and add R- 10.0 g of methylbenzylamine and hydrochloric acid adjust the pH of the reaction solution to about 8.0, then add 30.0 g of ω-transaminase and 0.08 g of pyridoxal phosphate (PLP), wherein the amino acid sequence of ω-transaminase is as shown in the sequence table SEQ ID NO .1 shown; keep the temperature at 25-30°C, pH around 8.0, react for 20 hours, the TLC raw material basically reacts completely, stop the reaction. Use concentrated hydrochloric acid to adjust the pH of the system to 2-3, add diatomaceous earth to filter after stirring, rinse the filter cake with water, extract impurities from the filtrate with dichloromethane, and adjust the pH of the water phase to above 10 with 30% sodium hydroxide aqueous solution , and then extracted with dichloromethane, the combi...
Embodiment 2
[0069] 1. Transamination reaction
[0070]
[0071] Add 10.0g of ethyl 3-(3-chloropropyl)-4-oxopyrrolidine-1-carboxylate (3-2) into 300mL of distilled water, add 15mL of DMSO to aid dissolution, and add 4.0g of Isopropylamine and hydrochloric acid adjust the pH of the reaction solution to about 9.0, then add 20.0 g of ω-transaminase and 0.05 g of pyridoxal phosphate (PLP), wherein the amino acid sequence of ω-transaminase is shown in the sequence table SEQID NO.2; Raise the temperature to 45-50°C, keep the pH at about 9.0, and after 12 hours of heat preservation reaction, the TLC raw material basically reacts completely, and the reaction is stopped. Use concentrated hydrochloric acid to adjust the pH of the system to 2-3, add diatomaceous earth to filter after stirring, rinse the filter cake with water, extract impurities from the filtrate with dichloromethane, and adjust the pH of the water phase to above 10 with 30% sodium hydroxide aqueous solution , and then extracted ...
Embodiment 3
[0076] 1. Transamination reaction
[0077]
[0078] Add 20.0g of 3-(1-benzyl-4-oxypyrrolidin-3-yl)propyl methanesulfonate (1-3) into 600mL of distilled water, add 10.0g of D-alanine and ammonia water while stirring Adjust the pH of the reaction solution to about 10.0, then add 40g of ω-transaminase and 0.10g of pyridoxal phosphate (PLP), wherein the amino acid sequence of ω-transaminase is as shown in the sequence table SEQ ID NO.3; the temperature is raised to 45- 50°C, keep the pH at about 10.0, and after 2 hours of heat preservation reaction, the TLC raw material basically reacted completely, and the reaction was stopped. Use concentrated hydrochloric acid to adjust the pH of the system to 2-3, add diatomaceous earth to filter after stirring, rinse the filter cake with water, extract impurities from the filtrate with dichloromethane, and adjust the pH of the water phase to above 10 with 30% sodium hydroxide aqueous solution , and then extracted with dichloromethane, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com