Cyanoguanidine prodrugs
A cyano group and group technology, applied in the field of preparation of drugs, can solve the problem of loss of antihypertensive activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
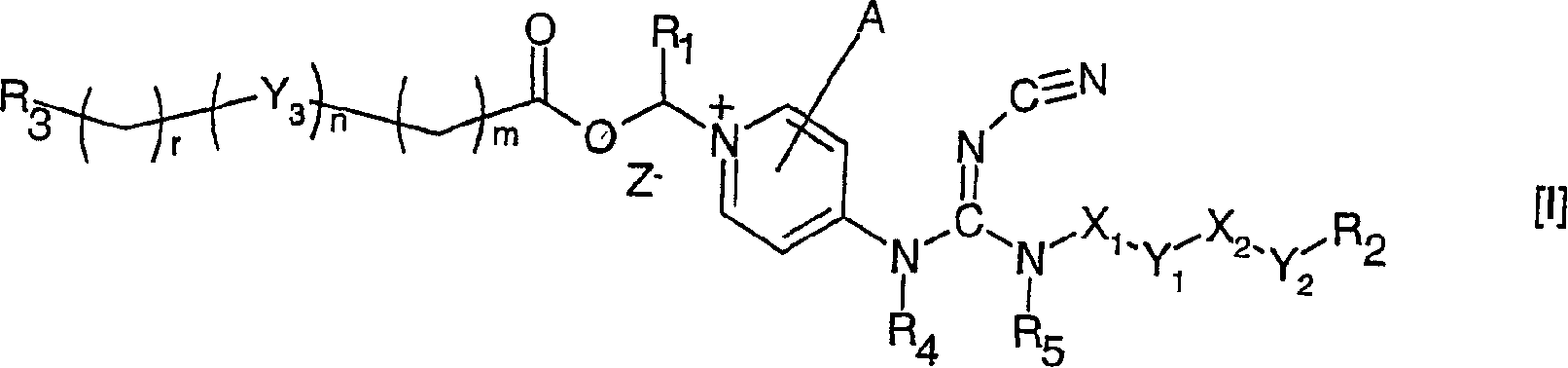
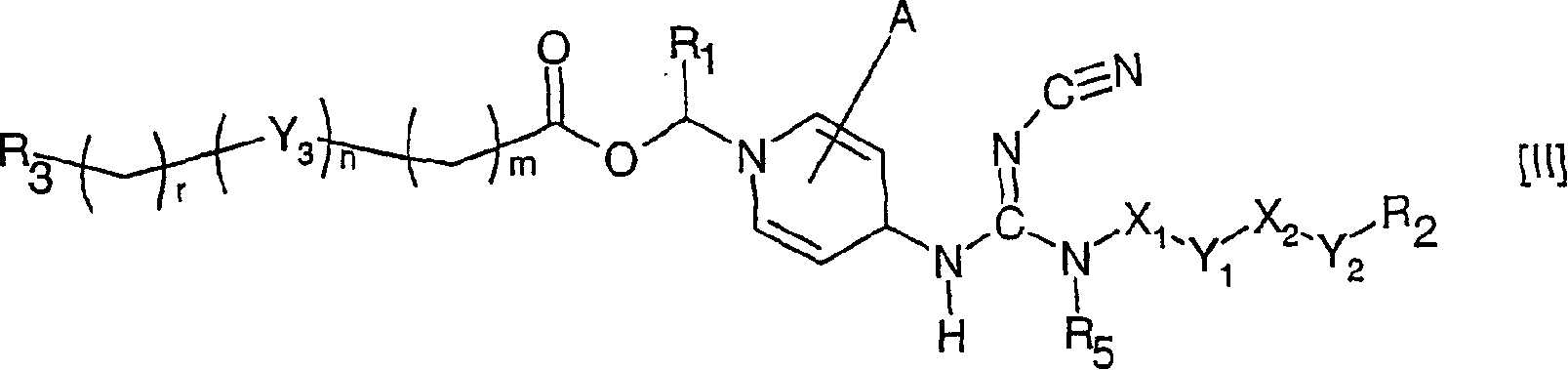
[0245] 1-[2-[2-(2-(2-Methoxyethoxy)-ethoxy)-ethoxy-carbonyloxymethyl Base] -4-[N'-cyano-N"-(6-(4-chlorophenoxy)-hexyl)-N-guanidino]-pyridinium chloride thing
[0246] N-(6-(4-chlorophenoxy)-hexyl)-N'-cyano-N"-(4-pyridyl)-guanidine (1.13g) was mixed with chloromethyl carbonate 2-(2-( A mixture of 2-methoxyethoxy)-ethoxy)-ethyl ester (1.95 g) was placed in a preheated oil bath at 100° C. After 15 minutes, a clear orange melt formed and after 45 minutes , the mixture was cooled to room temperature and EtOAc (5ml) was added. The desired compound crystallized and was isolated by filtration. Recrystallization from isopropanol gave an analytically pure sample.
[0247] 13 C NMR (DMSO) δ = 157.4, 155.0, 153.0, 144.9, 129.1, 123.9, 116.1, 115.0, 112.8, 80.2, 71.1, 69.6, 69.5, 68.0, 67.7, 67.6, 57.9, 42.2, 28.3, 25.7, 25.0
Embodiment 2
[0249] 1-[2-(2-Methoxyethoxy)-ethoxy-carbonyloxymethyl]-4-[N'-cyano- N”-(6-(4-chlorophenoxy)-hexyl)-N-guanidino]-pyridinium chloride
[0250] Following the procedure described in Example 1, but substituting chloromethyl carbonate 2-(2-methoxyethoxy)-ethyl ester for chloromethyl carbonate 2-(2-(2-methoxyethoxy) (yl)-ethoxy)-ethyl ester, the title compound was isolated as a well crystalline compound.
[0251] 13 C NMR (DMSO) δ = 157.4, 155.0, 153.0, 144.9, 129.1, 123.9, 116.1, 115.0, 112.7, 80.2, 71.1, 69.4, 68.0, 67.7, 67.6, 58.0, 42.2, 28.3, 25.7, 25.0
Embodiment 3
[0253] 1-[2-Methoxyethoxy)-carbonyloxymethyl]-4-[N’-cyano-N”-(6-(4-chloro Phenoxy)-hexyl)-N-guanidino]-pyridinium chloride
[0254]Following the procedure described in Example 1, but substituting chloromethyl 2-methoxyethyl carbonate for chloromethyl 2-(2-(2-methoxyethoxy)-ethoxy)-ethyl carbonate base ester, the title compound was isolated as crystalline compound.
[0255] 13 C NMR (DMSO) δ = 157.4, 155.0, 153.0, 144.8, 129.1, 123.9, 116.1, 115.0, 112.8, 80.2, 69.2, 67.8, 67.6, 57.9, 42.1, 28.3, 28.2, 25.7, 25.0
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com