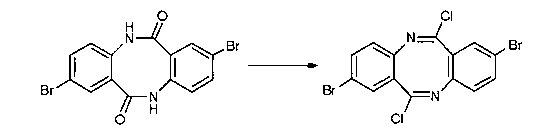
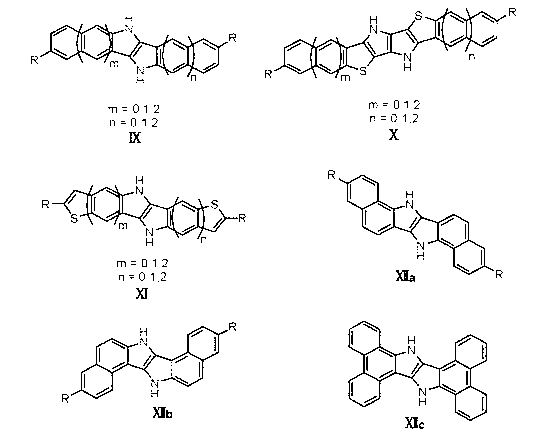
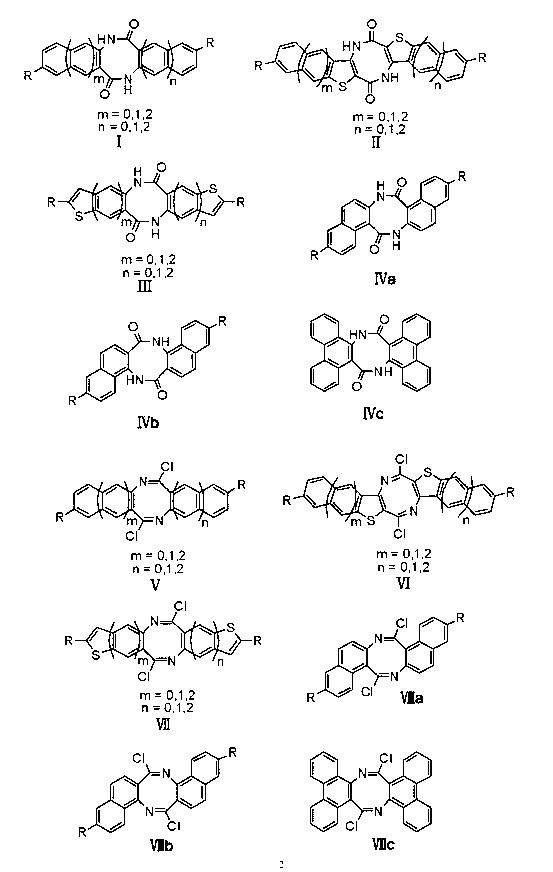
Method for preparing novel organic field effect transistor material
An organic field and transistor technology, which is applied in the field of preparation of new organic field effect transistor materials, can solve the problems of cumbersome synthesis methods and insufficient universality, and achieve the effect of simple and efficient synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] In the dry schlenk tube, under nitrogen protection, add 0.9g of diazocine compound, 15ml of dry chloroform, stir well, and cool to 0 o C, add 1.68g phosphorus pentachloride in three batches, naturally rise to room temperature, then heat to reflux, continue to stir for 4h, after the reaction is completed, cool to room temperature, solvent is concentrated under reduced pressure, flash column chromatography (silica gel, petroleum ether: Ethyl acetate = 10:1 rinse), to obtain 0.66 g of light yellow imidoyl chloride compound, yield 65%.
[0029] 1 H NMR (CDCl 3 ) δ: 7.38-7.34 (m, 4H); 7.15 (t, J=7.6Hz, 2H); 6.99 (d, J=8.1Hz, 2H). 13 C NMR (CDCl 3 ) δ: 156.3, 145.3, 131.6, 127.1, 126.1, 125.4, 122.0. IR (KBr, cm -1 ): 3160, 3034, 2900, 1649, 1598, 1481, 1439, 1397, 1216, 948, 755. Calculated HRMS(EI): C 14 h 8 Cl 2 N 2 274.0065, measured value: 297.0086[M+Na] + .
Embodiment 2
[0031]
[0032] Operation with reference to Example 1, yield 75%.
[0033] 1 H NMR (CDCl 3 ) δ: 7.51 (dd, J 1 =8.7Hz,J 2 =2.2Hz, 2H); 7.46 (d, J=2.2Hz,2H);
[0034] 6.88 (d, J=8.7Hz, 2H). 13 C NMR (CDCl 3 ) δ: 155.2, 144.0, 134.9, 129.7, 127.3, 123.8, 118.8. Calculated HRMS(EI): C 14 h 6 Br 2 Cl 2 N 2 429.8275, measured value: 452.8301[M+Na] + .
Embodiment 3
[0036]
[0037] Operation with reference to Example 1, yield 60%.
[0038] 1 H NMR (CDCl 3 ) δ: 7.88 (s, 2H); 7.77 (d, J=8.4Hz, 2H); 7.71 (d, J=8.4Hz, 2H), 7.49-7.47 (m, 2H), 7.43-7.41 (m, 4H ). 13 C NMR (CDCl 3 ) δ: 156.6, 141.5, 134.3, 130.2, 128.4, 128.3, 127.5, 126.7, 126.4, 118.9. IR (KBr, cm -1 ): 3054, 2928, 1654, 1594, 1495, 1389, 1174, 1114, 929, 772. Calculated HRMS(EI): C 22 h 12 Cl 2 N 2 374.0378, measured value: 397.0325[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com