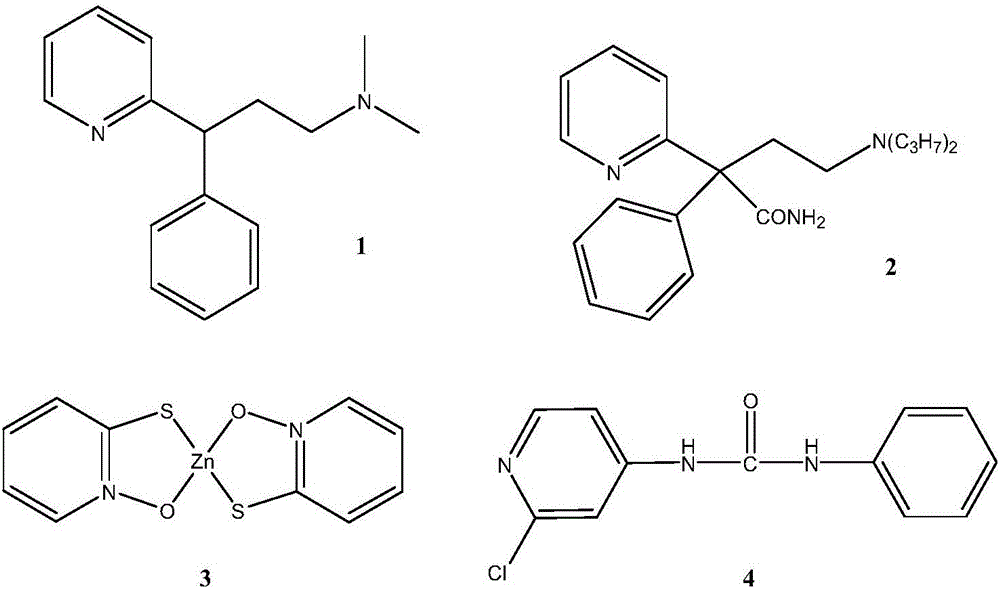
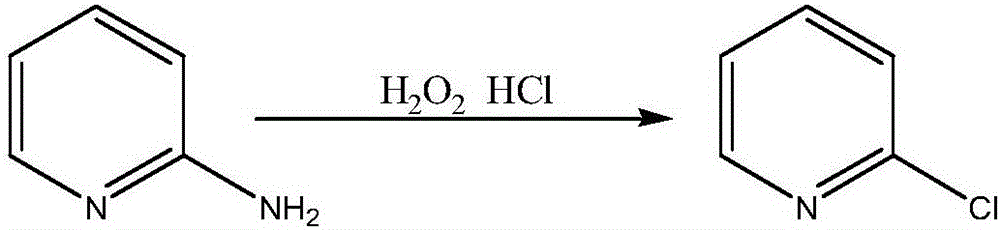Method for synthesizing 2-chloropyridine
A chloropyridine and pyridine technology, applied in the field of 2-chloropyridine synthesis, can solve the problems of low single-pass yield, many by-products, and high process requirements, and achieve the effect of safe and reliable process production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 79g of pyridine and 170g of dichloromethane into a 500mL container, stir for 15min, then add 95g of fluorine acetate dropwise at a rate of 3g / min for 30min, and react at 20°C for 4 hours; transfer the reaction mixture to 500mL In the container, carry out vacuum distillation under the conditions of temperature 70°C and vacuum degree 0.07Mpa. After no liquid is evaporated, the remaining material is 2-chloropyridine. The content of 2-chloropyridine was detected to be 94.5%, and the yield was 80.2%.
Embodiment 2
[0031] Add 79g of pyridine and 170g of dichloromethane into a 500mL container, stir for 15min, then dropwise add 100g of fluorine acetate at a rate of 4g / min, dropwise for 30min, and react at 20°C for 4 hours; transfer the reaction mixture to 500mL In the container, carry out vacuum distillation under the conditions of temperature 60°C and vacuum degree 0.07Mpa. After no liquid is evaporated, the remaining material is 2-chloropyridine. The content of 2-chloropyridine was detected to be 94.8%, and the yield was 80.8%.
Embodiment 3
[0033] Add 79g of pyridine and 170g of dichloromethane into a 500mL container, stir for 15min, then add 105g of fluorine acetate dropwise at a rate of 4g / min for 30min, and react at 20°C for 4 hours; transfer the reaction mixture to 500mL In the container, carry out vacuum distillation under the conditions of temperature 50°C and vacuum degree 0.07Mpa. After no liquid is evaporated, the remaining material is 2-chloropyridine. The content of 2-chloropyridine was detected to be 94.7%, and the yield was 81.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com