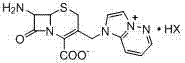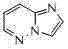Preparation method of cefozopran hydrochloride intermediate
A technology for cefozopram hydrochloride and its intermediates, which is applied in the field of preparation formula (I), can solve the problems of complicated operation, high cost of raw materials, long production cycle, etc., and achieve the effect of high yield and few adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] A kind of preparation method of cefozopram hydrochloride intermediate of the present invention, comprises the steps:
[0031] ① The chemical name of the intermediate of cefazolam hydrochloride: 7-amino-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl] Methyl]imidazo[1,2-b]pyridazinium salt, represented by the following general formula (I):
[0032]
[0033] Formula (I), wherein HX represents HI, HCl, H 2 SO 4 Wait;
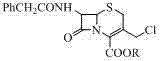
[0034] ② Using formula (II) 7-phenylacetamido-3-chloromethyl cephalosporanate (GCLE) as the starting material, first activate the C-3 position with iodine, potassium iodide or sodium iodide hydrate and then combine with the formula ( Ⅲ) Imidazo[1,2-b]pyridazine is reacted, and after post-treatment, the compound of formula (Ⅳ) is obtained;
[0035]
[0036] Formula (II); wherein R is p-methoxybenzyl or benzhydryl; Ph is phenyl;
[0037]
[0038] Formula (Ⅲ);
[0039]
[0040] Formula (Ⅳ);
[0041] ③ The compou...
Embodiment 1
[0049] step:
[0050] ①Under nitrogen protection, dissolve 97g of potassium iodide in 50ml of deionized water, add 500ml of acetone, stir, cool down to 5°C in an ice-water bath, add 47g of 7-phenylacetamido-3-chloromethyl cephalosporanic acid benzyl, Avoid light reaction, keep temperature 5-8 o C was reacted for 10 hours; acetone was evaporated under reduced pressure, 100ml of deionized water was added, extracted with dichloromethane, and the organic layer was washed with 1mol / L sodium thiosulfate solution, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 57g of light yellow oil, dissolve the oil in dichloromethane, stir, add 15g of imidazopyridazine, 25-28 o C reaction for 10 hours, concentrated dichloromethane under reduced pressure to the end, added anhydrous methanol, precipitated solid, filtered, washed and dried. Obtain light yellow solid 7-phenylacetamido-2-methoxybenzyl carboxylate-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3...
Embodiment 2
[0054] ① Under the protection of nitrogen, dissolve 25g of sodium iodide in 20ml of water, add to 480ml of acetone, stir, cool the ice water to 0°C, add 50g of 7-phenylacetamido-3-chloromethyl cephalosporanic acid methoxybenzyl ester , Avoid light reaction, keep warm at 0-5°C for 6 hours. Evaporate acetone under reduced pressure, then add dichloromethane, 25g imidazopyridazine at 25-28 o C was reacted for 8 hours, washed with 1mol / L sodium thiosulfate solution, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, added anhydrous methanol, precipitated solid, filtered, washed, dried to obtain a light yellow solid 7 -Phenylacetylamino-2-methoxybenzyl carboxylate-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]imidazo [1,2-b]Pyridazinium iodide (Formula II) 66.3g, HPLC detection purity 99%.
[0055] ② Put 63g of phosphorus pentachloride and 1000ml of dichloromethane into the reaction bottle, cool down the reaction solution to -10°C, con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com