A kind of preparation method of 5-flucytosine suitable for industrialized production
A technology for flucytosine and fluoropyrimidine, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of large hidden dangers in production safety, high rate of three wastes and high cost of raw materials, and achieves the effects of less pollution of three wastes, convenient post-processing and stable product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
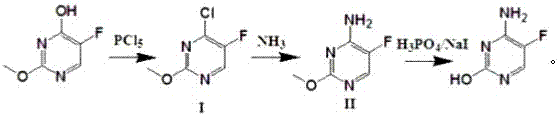
[0023] Example 1 Preparation of 2-methoxy-4-chloro-5-fluoropyrimidine (I)
[0024] 2-Methoxy-5-fluorouracil (144g, 1mol), toluene 500g, N,N-dimethylaniline (110g, 0.92mol), temperature controlled at 35-40°C, phosphorus pentachloride (412g , 2mol), each batch is about 20g, one batch every 3 minutes, and the addition is completed in about 1 hour. After the addition, the temperature was raised to 85°C, and stirring was continued for 3 hours. After cooling to room temperature, the reaction solution was poured into 1500 g of ice water, stirred for 30 minutes, separated, and the organic phase was spin-dried to obtain 2-methoxy-4-chloro- The crude product of 5-fluoropyrimidine (I) was 167 g, no further purification was needed; the aqueous phase was spun under reduced pressure until no water was evaporated to obtain an acidic aqueous phase concentrate for later use.
example 2
[0025] Example 2 Preparation of 2-methoxy-4-amino-5-fluoropyrimidine (Ⅱ)
[0026] Put 167g of the crude product of 2-methoxy-4-chloro-5-fluoropyrimidine (I) obtained in Example 1 into a 1L autoclave, add 350g of ammonia water, after sealing, heat up to 100°C, pressure 0.7Mpa, stir 2 hours. Cool the reaction solution to 0-5°C, precipitate solids, filter, and dry the filter cake to obtain 128 g of white solids (the total yield of two steps is 89.5%), and the ammonia filtrate is used for later use.
example 3
[0027] Preparation of Example 3 5-fluorocytosine
[0028] The acidic aqueous phase concentrate obtained in Example 1, 2-methoxy-4-chloro-5-fluoropyrimidine (I) (128g, 0.895mol) and sodium iodide (6.7g, 0.045mol) were stirred at 130°C After 5 hours, after cooling to room temperature, the ammonia water filtrate in Example 2 was added dropwise to the reaction solution until pH=8.3, a large amount of solids were precipitated, filtered, the filter cake was recrystallized with water, and white 5-fluorocytosine was obtained after drying 98g of solid (84.9% yield, 77.7% molar yield of the total route).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com