Preparation method of eplerenone
A technology of eplerenone and oxo generation, which is applied in the field of medicine, can solve the problems of poor appearance of the final product and dark color of the reaction solution, and achieve the effects of easy industrial production, good product quality and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
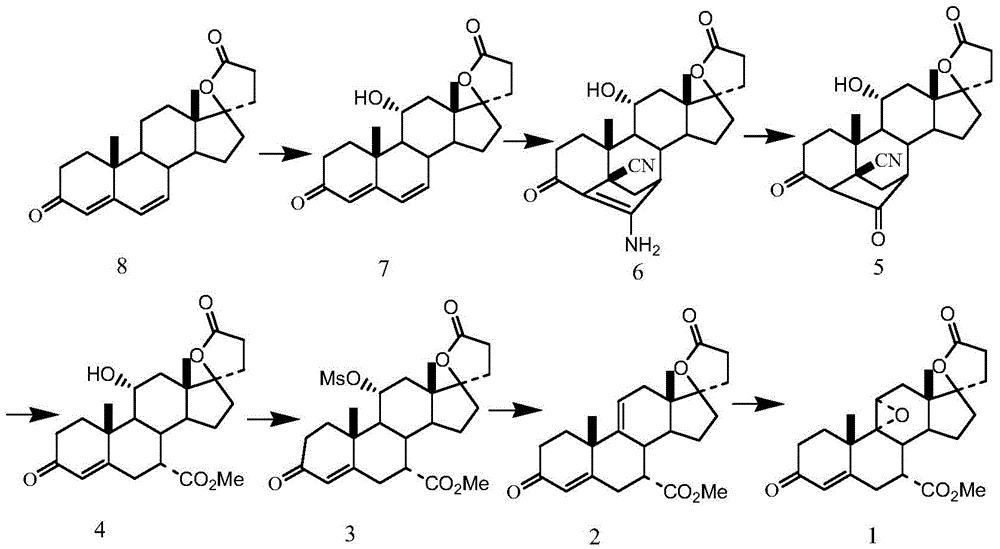
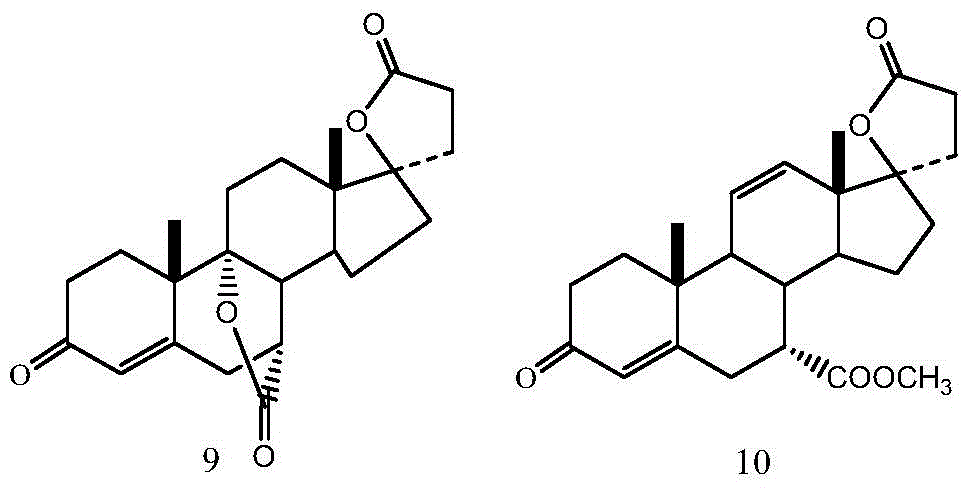
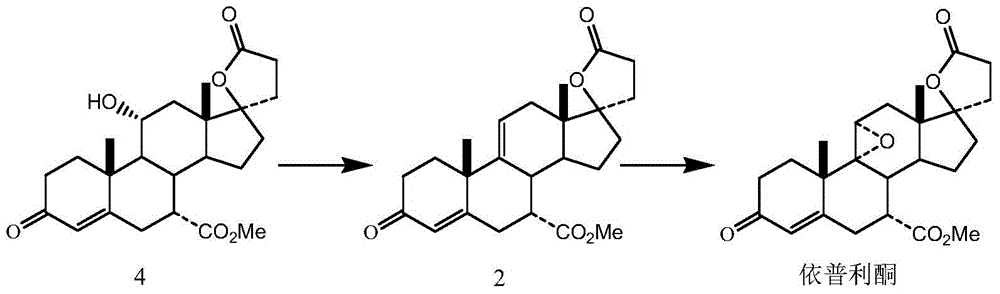
[0034] (1) Preparation of 17α-pregna-4,9(11)-diene-7α,21-dicarboxylic acid-17β-hydroxy-3-oxo-γ-lactone, 7-methyl ester (compound 2):
[0035] Put 20g of 17α-pregn-4-ene-7α, 21-dicarboxylic acid-11α, 17β-dihydroxy-3-oxo-γ-lactone-7-methyl ester (compound 4) into the four-necked reaction flask and tetrahydrofuran 150mL, lower the temperature to 10°C, add 90mL of boron trichloride in dichloromethane solution (equivalent to 10.55g of boron trichloride) dropwise into the reaction flask under stirring, and drop it in 30 minutes. Then, the reaction solution was lowered to 10° C., 12 g of phosphorus pentachloride was added, and the reaction was continued with stirring. The reaction was detected by TLC until the spots of the raw materials disappeared, and the reaction was stopped.
[0036] Cool down to 0°C, slowly add 60 mL of deionized water, and control the temperature of the reaction solution during the addition process to not exceed 20°C. After stirring for 10 minutes, 250 mL of d...
Embodiment 2
[0043] (1) Preparation of 17α-pregna-4,9(11)-diene-7α,21-dicarboxylic acid-17β-hydroxy-3-oxo-γ-lactone, 7-methyl ester (compound 2):
[0044] Put 20g of 17α-pregn-4-ene-7α, 21-dicarboxylic acid-11α, 17β-dihydroxy-3-oxo-γ-lactone-7-methyl ester (compound 4) into the four-necked reaction flask and 150 mL of tetrahydrofuran, cooled to 0°C, and 25 mL of boron trifluoride ether solution (equivalent to 6.2 g of boron trifluoride) was added dropwise with stirring. After the dropwise addition, the temperature of the reaction solution was lowered to 0° C., 30 g of phosphorus pentachloride was added, and the stirring was continued for 10 minutes. The reaction was detected by TLC until the spots of the raw materials disappeared, and the reaction was stopped.
[0045] After the reaction was completed, the temperature was lowered to 0°C, and 60 mL of deionized water was slowly added, and the temperature of the reaction solution was controlled not to exceed 20°C during the addition process....
Embodiment 3
[0052] (1) Preparation of 17α-pregna-4,9(11)-diene-7α,21-dicarboxylic acid-17β-hydroxy-3-oxo-γ-lactone, 7-methyl ester (compound 2):
[0053] Put 20g of 17α-pregn-4-ene-7α, 21-dicarboxylic acid-11α, 17β-dihydroxy-3-oxo-γ-lactone-7-methyl ester (compound 4) into the four-necked reaction flask and 150 mL of tetrahydrofuran, cooled to 5°C, and 136 mL of boron trichloride in dichloromethane solution (equivalent to 16.0 g of boron trichloride) was added dropwise with stirring. After the dropwise addition, the temperature of the reaction solution was lowered to 5° C., 16 g of phosphorus pentachloride was added, and the stirring was continued for 10 minutes. The reaction was detected by TLC until the spots of the raw materials disappeared, and the reaction was stopped.
[0054] After the reaction was completed, the temperature was lowered to 0°C, and 60 mL of deionized water was slowly added, and the temperature of the reaction solution was controlled not to exceed 20°C during the ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com