Preparation of phosphorus pentafluoride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
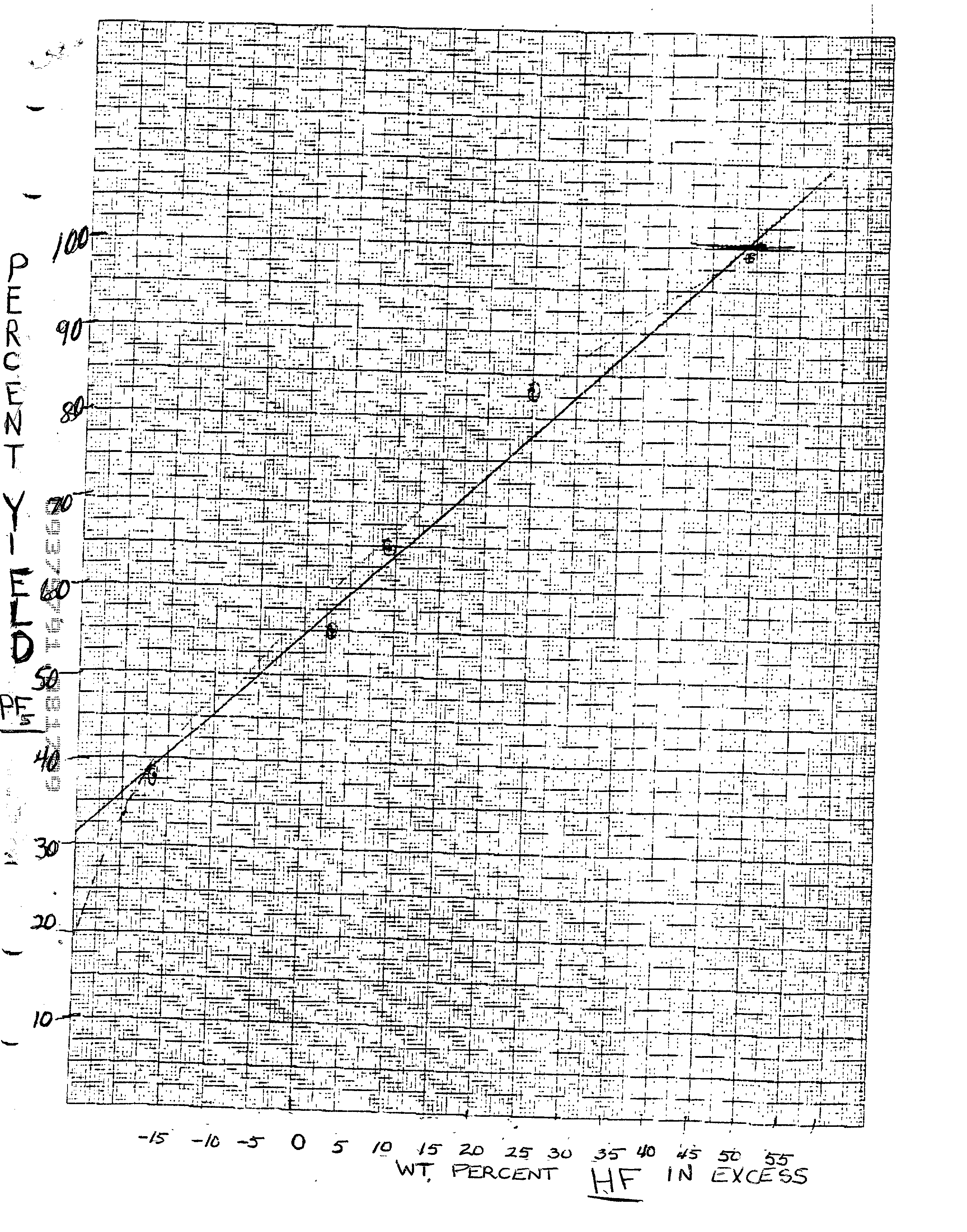
[0057] 25% excess HF added
[0058] To 1044 g. (5.0 moles) of the 70% HPF.sub.6 solution prepared according to the first step described in Example 1, were added 150 g. liquid anhydrous HF to provide a 25% excess of HF in the solution. To this mixture were slowly added 1781 g. (14.5 moles) fuming sulfuric acid (65% SO.sub.3) over three hours maintaining the temperature below 32.degree. C. The PF.sub.5 was passed through series of traps and into preweighed cold ether as described. After the addition was completed, the temperature was gradually raised to 152.degree. C. to drive the excess HF to the traps. The recovered PF.sub.5 weighed 512 g which corresponds to a yield of 83 wt. %. A total of 278 g. of HF distillate from the traps was collected for use in the next run.
example 3
[0059] Addition of previous run HF distillate (equivalent to about 49% HF excess)
[0060] The 278 g. HF distillate from the ice traps in Example 2 is added to 993 g. (7.6 moles) 70% HPF.sub.6 as prepared in the first step of Example 1 with cooling to maintain the temperature below 25.degree. C. Then 1587 g. (12.9) fuming sulfuric acid (65% SO.sub.3) were added over three hours while maintaining the temperature of the solution under 32.degree. C. When the addition was completed, the PF.sub.5 was treated as before and trapped in cold diethyl ether. The yield was 580 g. which is 99 wt. %. The HF distillate weighed about 280 g. and could be recycled into the next run.
example 4
[0061] Added 316 g HF distillate from previous runs to 1003 g. 70% HPF.sub.6 prepared as in Example I. Then added 1306 g. fuming sulfuric acid (65% SO.sub.3) were added to the solution slowly maintaining temperature below 29.degree. C. The solution was warmed up to 155.degree. C. The PF.sub.5 and HF gases were passed through the ice trap and dry ice traps as in previous examples. The PF.sub.5 was trapped in 723 g. diethyl ether at 0.degree. C. The net weight of pure PF.sub.5 was 566 g. (or 96 wt. % yield) about 30 g. POF.sub.3 and 364 g HF distillate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com