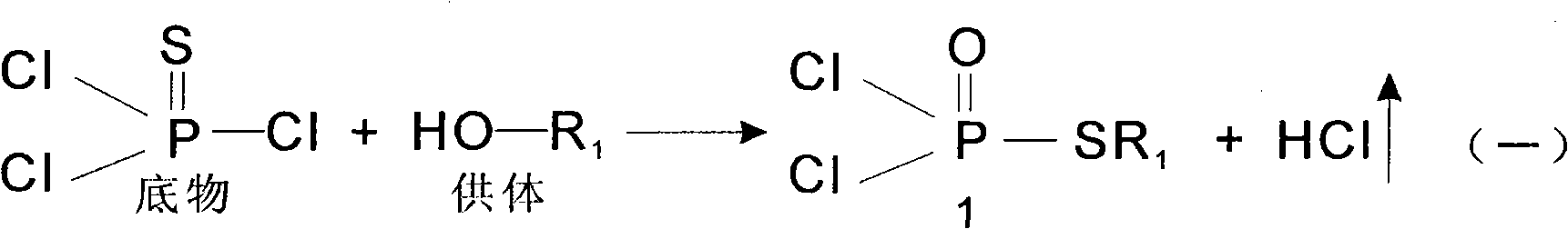
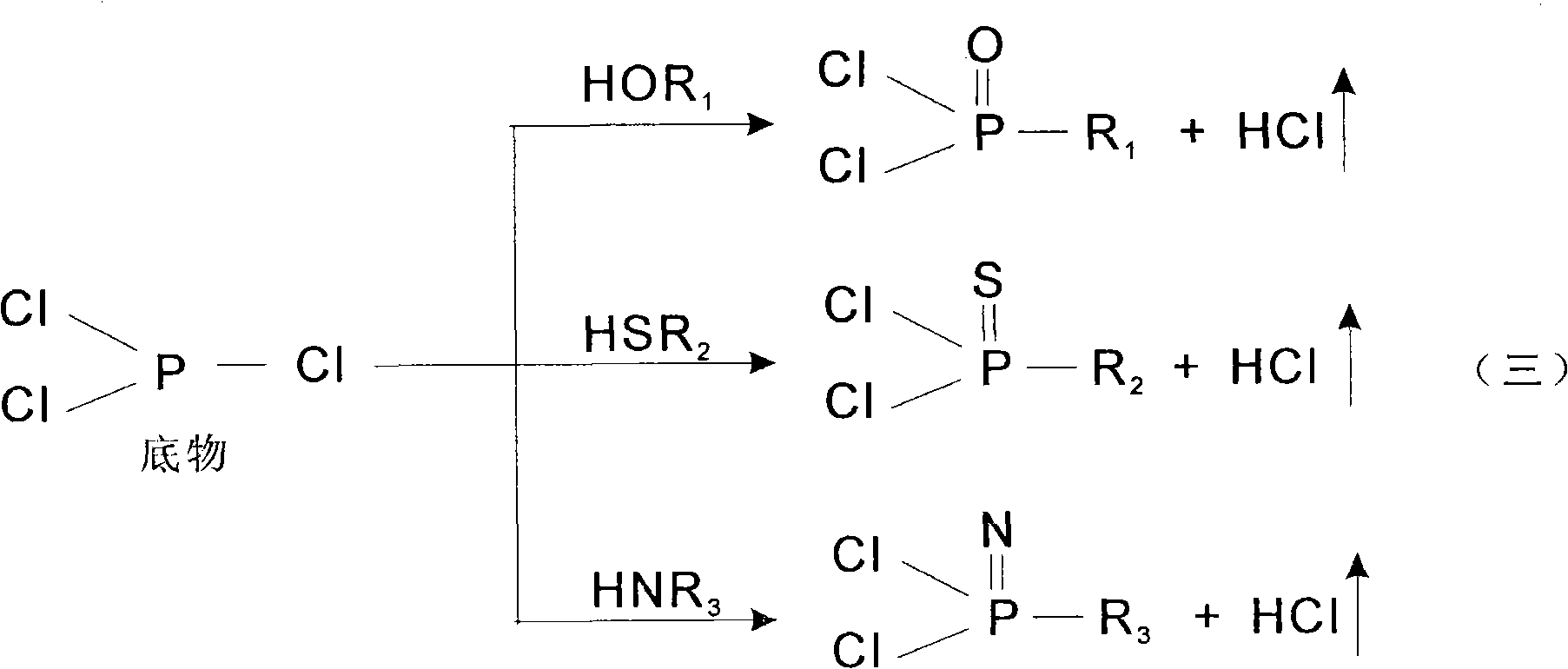
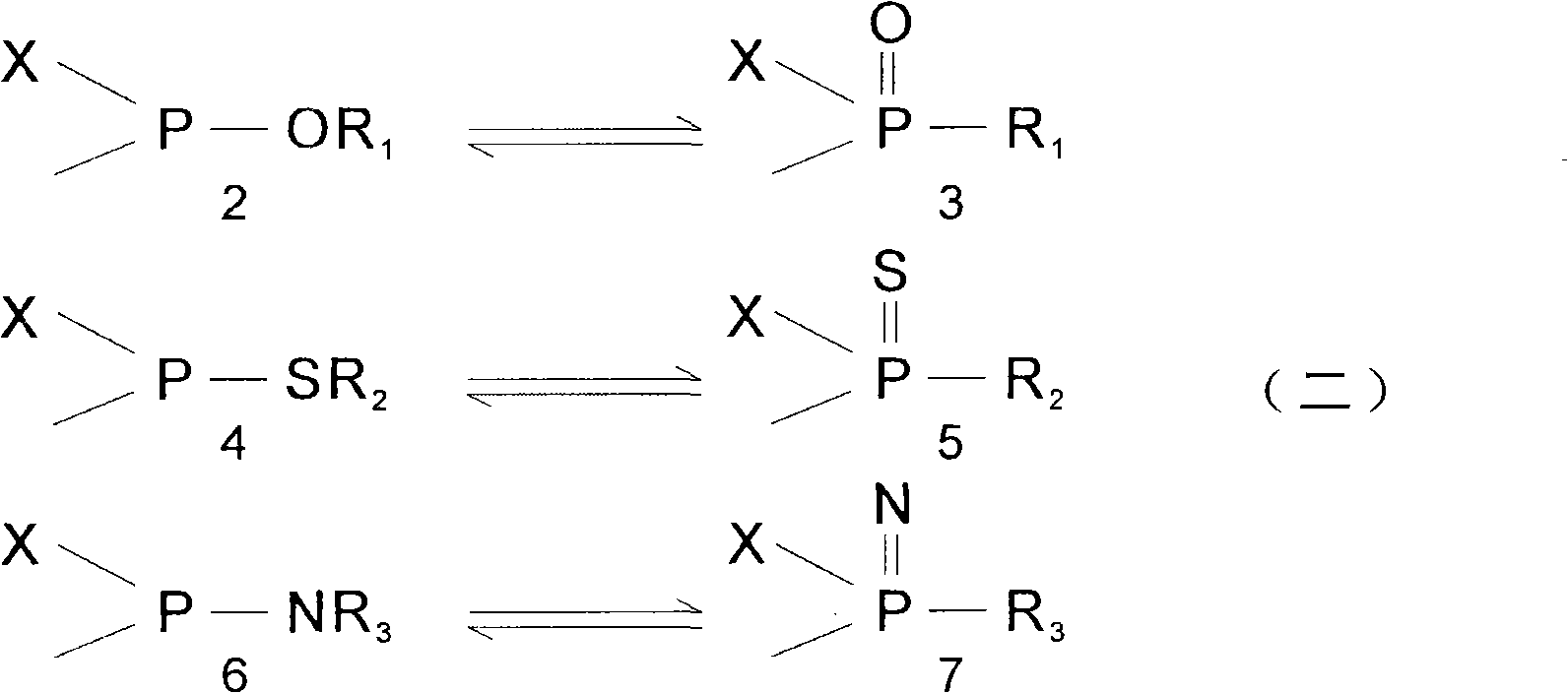Isomerization reaction
A technology of isomerization reaction and reaction, which is applied in application, biocide, plant growth regulator, etc., can solve the problem of high cost of wastewater treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Throw 100 grams of 98% phosphorous trichloride and 100 grams of anhydrous n-hexane into a four-necked reaction bottle, start stirring, heat up to reflux, add 28-37 g of anhydrous methanol dropwise, HCl gas continuously overflows, and reflux reaction for 16 After ~30 hours, when the HCl gas basically does not overflow, it is the end of the reaction. Cool down to normal temperature, let stand for more than 7 hours, divide into two layers, and separate the lower layer, which is the intermediate 10 compound. The average yield is 90 grams, the content is 98%,
[0125] Use n-hexane (or N, N-dimethylformamide) to prepare a 10-20% solution of compound 10, put it into a reaction bottle, control the temperature from 40°C to reflux, slowly and evenly add 34 grams of 99% acetamide solid, Constantly have HCl gas to overflow, react after 14 hours, basically do not have HCl gas to overflow and be the reaction end point, cool down to normal temperature, leave standstill for 7 hours, a ...
Embodiment 2
[0127] Throw 250 grams of anhydrous trimethyl phosphate (or N,N-dimethylformamide) into the reaction bottle, start stirring, control the temperature at 20-40°C, slowly add 50 grams of 98% glycine hydrochloride, and dissolve all After the solution becomes clear, slowly add 13 grams of paraformaldehyde, stir the reaction at this temperature until the solution is clear and transparent, cool down to about 15°C, add 61 grams of 98% phosphorus trichloride dropwise, , under the protection of isolated air or nitrogen, stirred and reacted for 14-20 hours, a large amount of solids precipitated, filtered and dried at room temperature and under the condition of isolated air to obtain 88 g of compound 11, with a content of 98%; the mother liquor was applied mechanically;
[0128] Put 88 grams of compound 11 into the reaction bottle, start stirring, slowly add 250 grams of 50-70% methanol aqueous solution, control the temperature at 40°C to reflux, react for 2-8 hours, then distill, and when...
Embodiment 3
[0130] 0.03mol of La(NO 3 ) 3 ·6H 2 O and GdCl 3 ·6H 2 O catalyst was added to 1mol of 3-hydroxypropionaldehyde and 1mol of NaCN in aqueous solution, stirred at room temperature for 15 minutes and all dissolved, then slowly added 1mol of formamide (or acetamide or saturated methanol / ammonia solution) and reacted at 40°C After 3 hours, the reaction solution was concentrated, and the residue was separated through a silica gel column (cycloethane-ethyl acetate) to obtain 104 g of compound 12 liquid, with a content of 98%;
[0131] This 104 grams of compound 12 is dropped into excess (300 grams) of phosphorus trichloride, or dropped into an equimolar compound of 9, the temperature is controlled below 20°C, the reaction is stirred, and the liquid chromatography normalization method is used to test the reaction system When the content of compound 12 is less than 1%, it is the end of the reaction, a large amount of solids are precipitated, filtered, and dried to obtain an average...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com