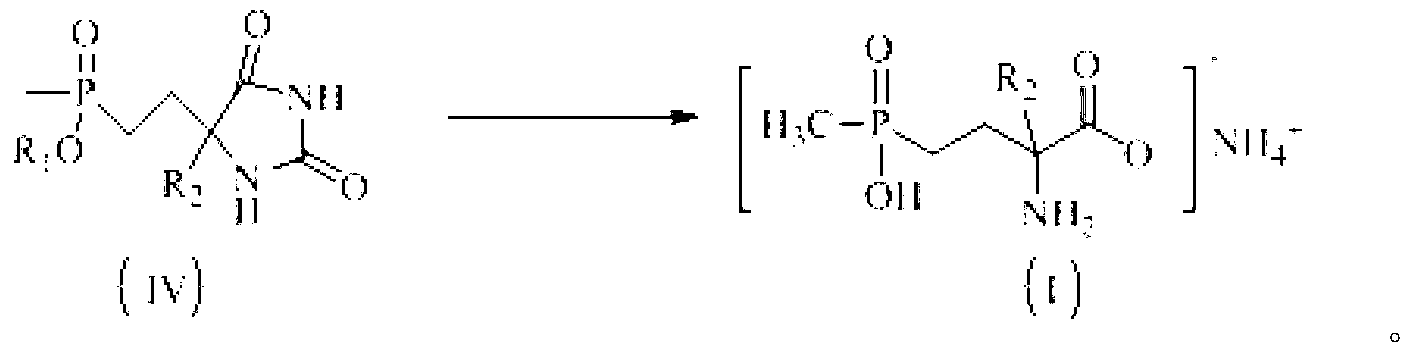
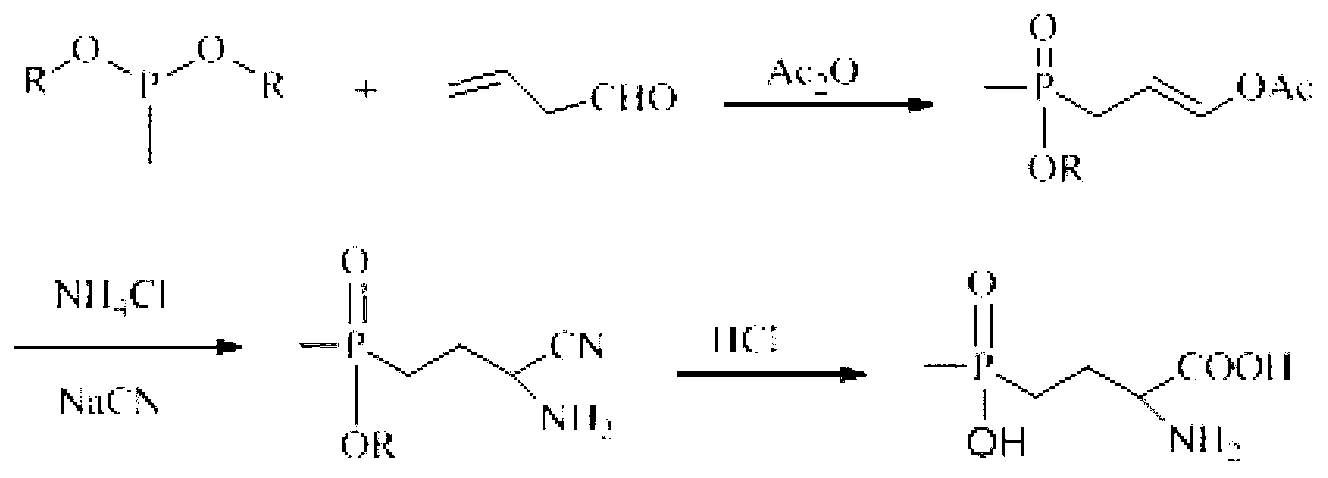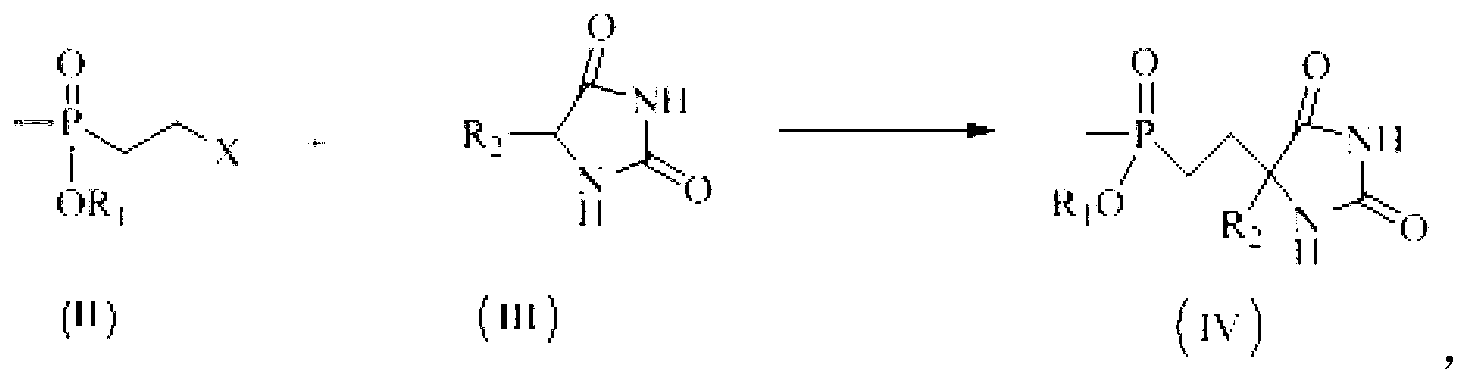Preparation method of glufosinate-ammonium and derivatives thereof
A derivative, glufosinate-ammonium technology, applied in the field of preparation of glufosinate-ammonium and its derivatives, can solve the problems of high environmental protection pressure, short reaction process, high boiling point, etc., to avoid the use of sodium cyanide, mild reaction conditions, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of 5-(2-(methylethoxyphosphono)ethyl)hydantoin
[0028] Add 12.01g (0.12mol) of hydantoin, 10.2g (0.15mol) of sodium ethoxide, and 90mL of ethanol into a 250mL three-necked flask, and stir at room temperature for 20min. After completely dissolving, add methyl-2-bromoethylphosphonic acid dropwise Ethyl ester 21.4g (0.10mol), after the dropwise addition was completed, the temperature was raised to 80°C to react for 5h, cooled to room temperature, the solid was removed by suction filtration, the solvent was removed by rotary evaporation, 100mL ethanol was added to dissolve and filter, and 5-(2-( Methylethoxyphosphono)ethyl)hydantoin crude product 20.88g, yield: 89.2%, content: 86.4%. 1 H NMR (D 2 O,300MHz)δ:1.212~1.245(m,3H);1.513(d,J=13.6Hz,3H);1.672~1.910(m,4H);3.928~3.976(m,2H);4.182(t,J =5.4Hz, 1H).
Embodiment 2
[0029] Example 2: Preparation of 5-(2-(methylethoxyphosphono)ethyl)hydantoin
[0030] Add 12.01g (0.12mol) of hydantoin, 10.2g (0.15mol) of sodium ethoxide, and 90mL of ethanol into a 250mL three-necked flask, and stir at room temperature for 20min. After completely dissolving, add methyl-2-bromoethylphosphonic acid dropwise 21.4g (0.10mol) of ethyl ester, after the dropwise addition was completed, the temperature was raised to 40°C to react for 5h, cooled to room temperature, the solid was removed by suction filtration, the solvent was removed by rotary evaporation, and 100mL ethanol was added to dissolve and filter, and 5-(2-( Methylethoxyphosphono)ethyl)hydantoin crude product 13.68g, yield: 58.4%, content: 56.6%.
Embodiment 3
[0031] Example 3: Preparation of 5-(2-(methylethoxyphosphono)ethyl)hydantoin
[0032] Add 12.01g (0.12mol) of hydantoin, 10.2g (0.15mol) of sodium ethoxide, and 90mL of ethanol into a 250mL three-necked flask, and stir at room temperature for 20min. After completely dissolving, add methyl-2-bromoethylphosphonic acid dropwise Ethyl ester 21.4g (0.10mol), after the dropwise addition was completed, the temperature was raised to 80°C for 3h, cooled to room temperature, the solid was removed by suction filtration, the solvent was removed by rotary evaporation, and 100mL ethanol was added to dissolve and filter, and 5-(2-( Methylethoxyphosphono)ethyl)hydantoin crude product 18.36g, yield: 78.4%, content: 76.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com