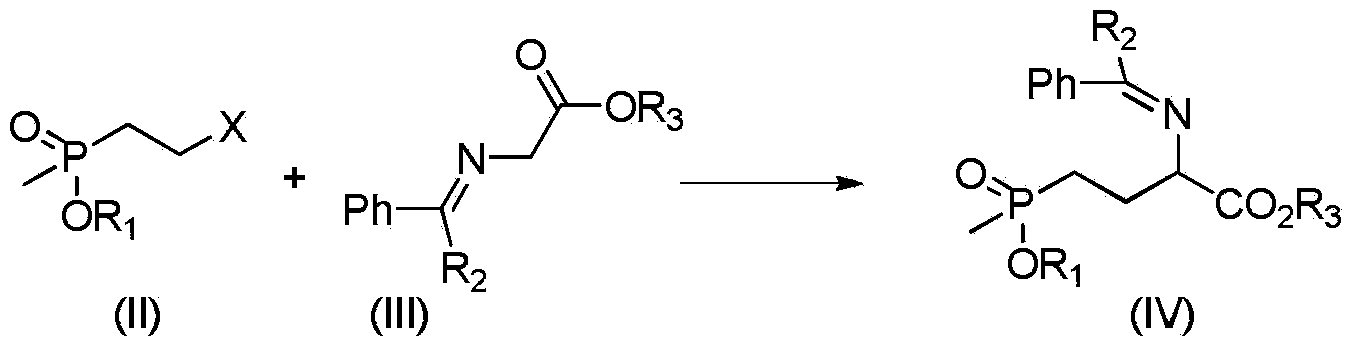
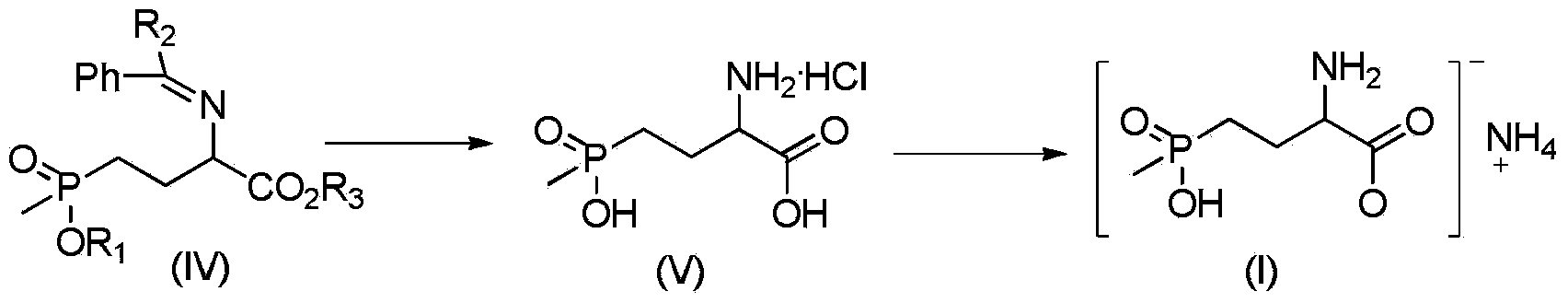
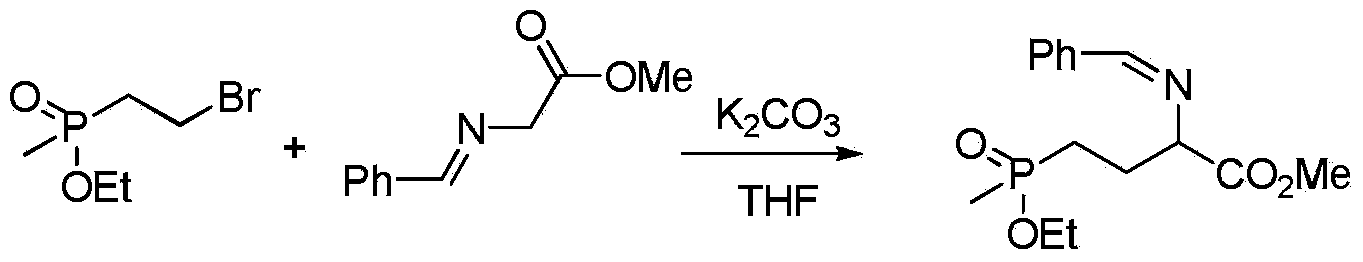
Preparation method of glufosinate-ammonium
A technology of glufosinate-ammonium and methylphosphonic acid, which is applied in the field of synthesis of glufosinate-ammonium, can solve the problems of cumbersome purification process, high environmental requirements, and difficult monitoring, and achieve the effect of high purity, high yield, and easy monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] step 1
[0021]
[0022] In a 250mL three-necked flask, add 10.6g (0.05mol) methyl (2-bromoethyl) ethyl phosphonate, 8.85g (0.05mol) phenylmethylene glycine methyl ester, 8.34g (0.06mol) of potassium carbonate and 100mL tetrahydrofuran, heated to reflux, liquid phase monitoring reaction until the reaction of phenylmethylene glycine methyl ester was completed, cooled, filtered, added 50mL dichloromethane after the filtrate was precipitated, washed with water until neutral, and the organic layer was spin-dried to obtain 2 - 14.3 g of phenylmethyleneamino-4-ethoxy(methyl)phosphonobutanoic acid methyl ester, yield 87.6%.
[0023] 1 H NMR (500MHz, CDCl 3 ):8.31(s,1H,CH),7.42-7.79(m,5H,5×Ar-H),4.36(t,1H,CH),4.22(q,2H,OCH 2 ),3.84(s,3H,OCH 3 ),1.94-2.02(m,2H,CH 2 ),1.54-1.69(m,2H,CH 2 ), 1.38(t,3H,CH 3 ),1.28(d,3H,J=3.8Hz,CH 3 ).
[0024] step 2
[0025]
[0026] Add 20 g (0.0615 mol) of 2-phenylmethyleneamino-4-ethoxy (methyl) phosphonobutanoic acid methyl est...
Embodiment 2
[0032] step 1
[0033]
[0034] In a 250mL three-necked flask, add 10.6g (0.05mol) ethyl methyl (2-bromoethyl) phosphonate, 13.35g (0.05mol) ethyl dibenzylidene glycine, 3.36g (0.06mol) potassium hydroxide ) and 100mL tetrahydrofuran, heat up to reflux, monitor the reaction in the liquid phase until the reaction of dibenzylidene glycine ethyl ester is completed, cool, filter, add 50mL chloroform after the filtrate is precipitated, wash with water until neutral, and spin dry the organic layer 16.7 g of ethyl 2-diphenylmethyleneamino-4-ethoxy(methyl)phosphonobutyrate was obtained, with a yield of 83.7%.
[0035] 1 H NMR (500MHz, CDCl 3 ):7.67(dd,2H,2×Ar-H),7.28-7.49(m,6H,6×Ar-H),7.16-7.20(m,2H,2×Ar-H),4.43(q,2H ,OCH 2 ),4.28(t,1H,CH),4.22(q,2H,OCH 2 ),1.98-2.08(m,2H,CH 2 ),1.59-1.72(m,2H,CH 2 ), 1.44(t,3H,CH 3 ), 1.41(t,3H,CH 3 ),1.30(d,3H,J=4.2Hz,CH 3 ).
[0036] step 2
[0037]
[0038] Add 15g (0.0374mol) ethyl 2-diphenylmethyleneamino-4-ethoxy (methyl) phosph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com