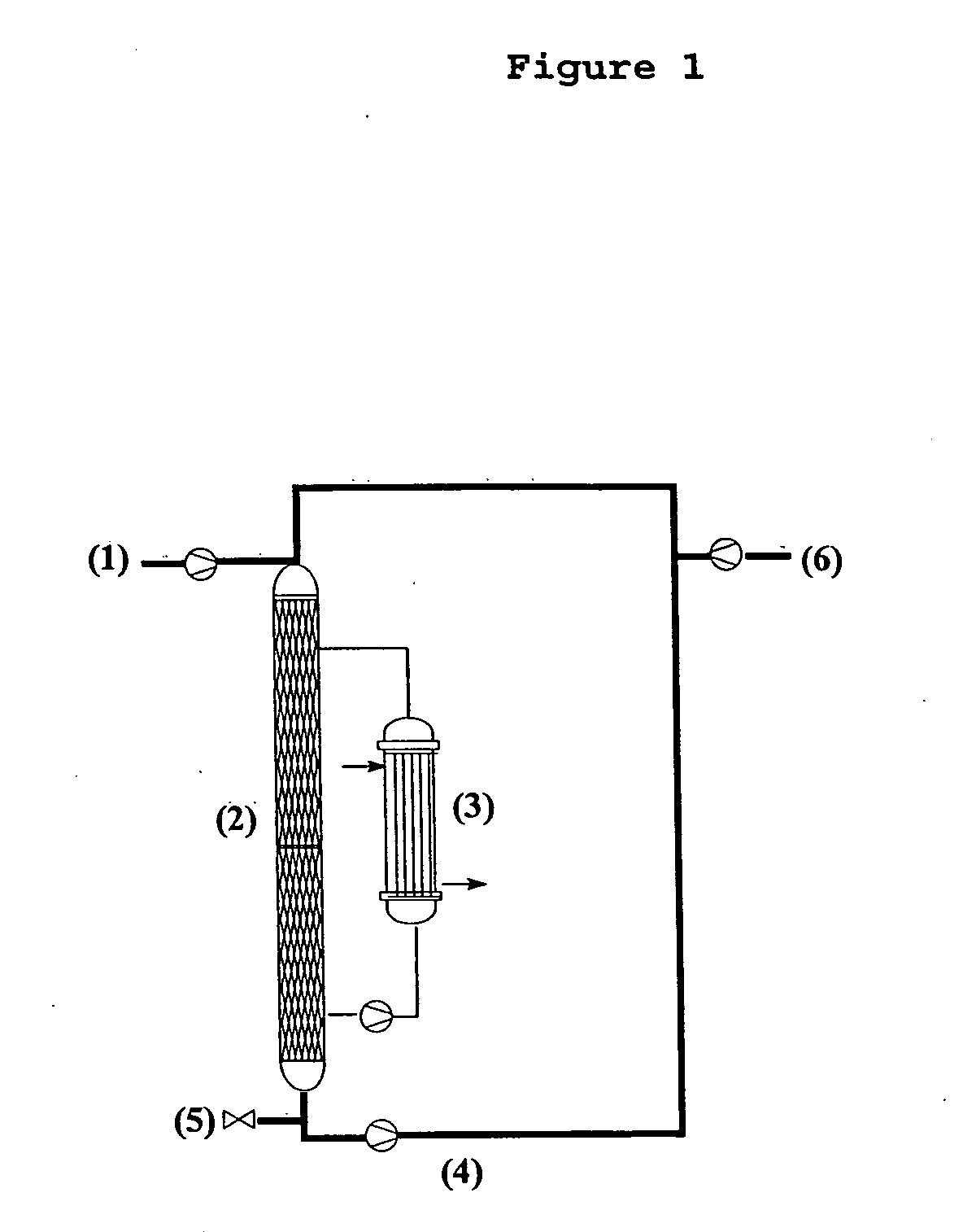
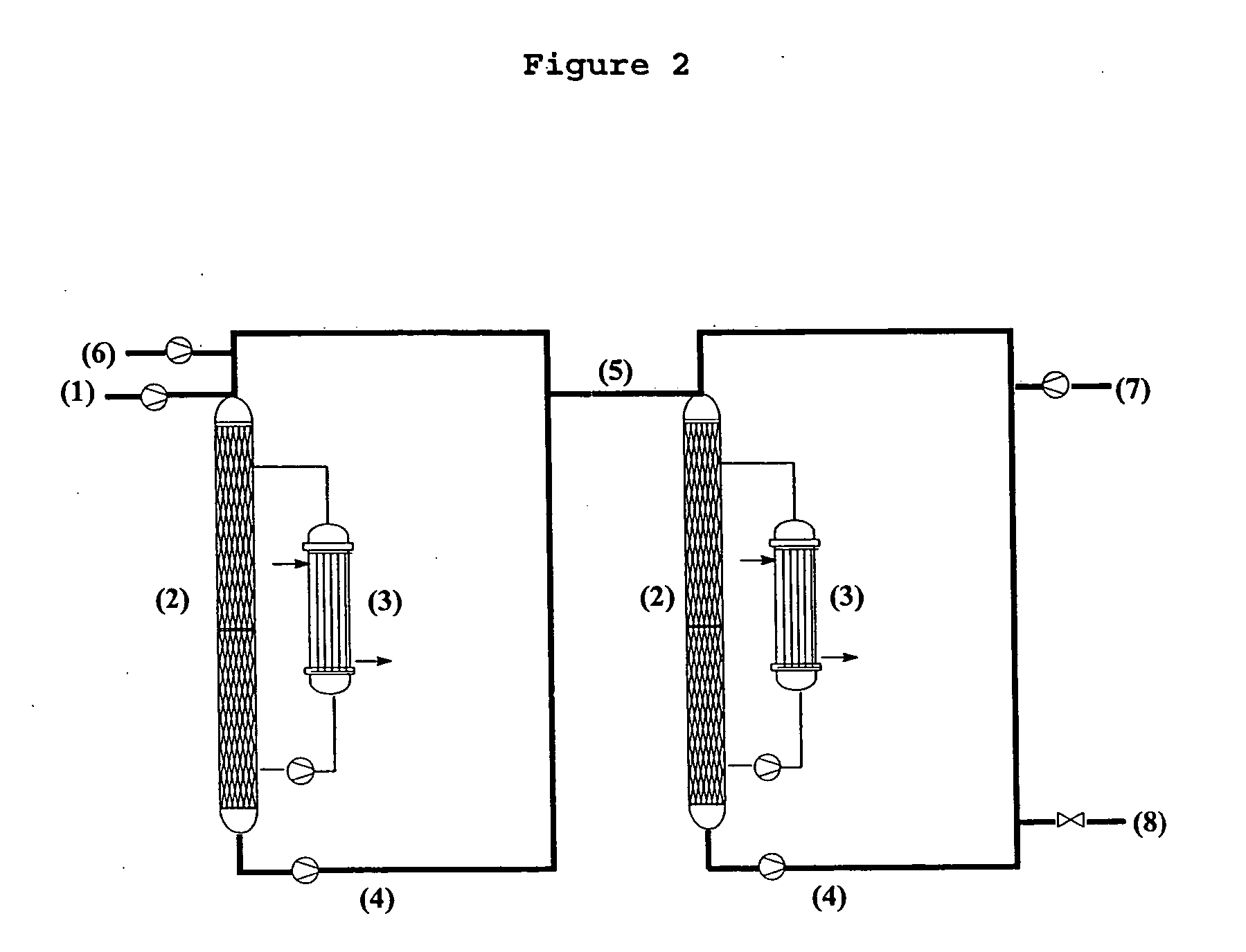
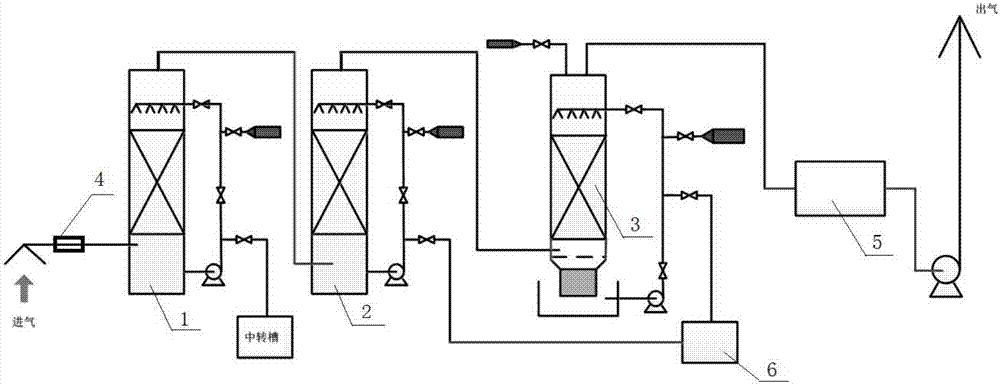
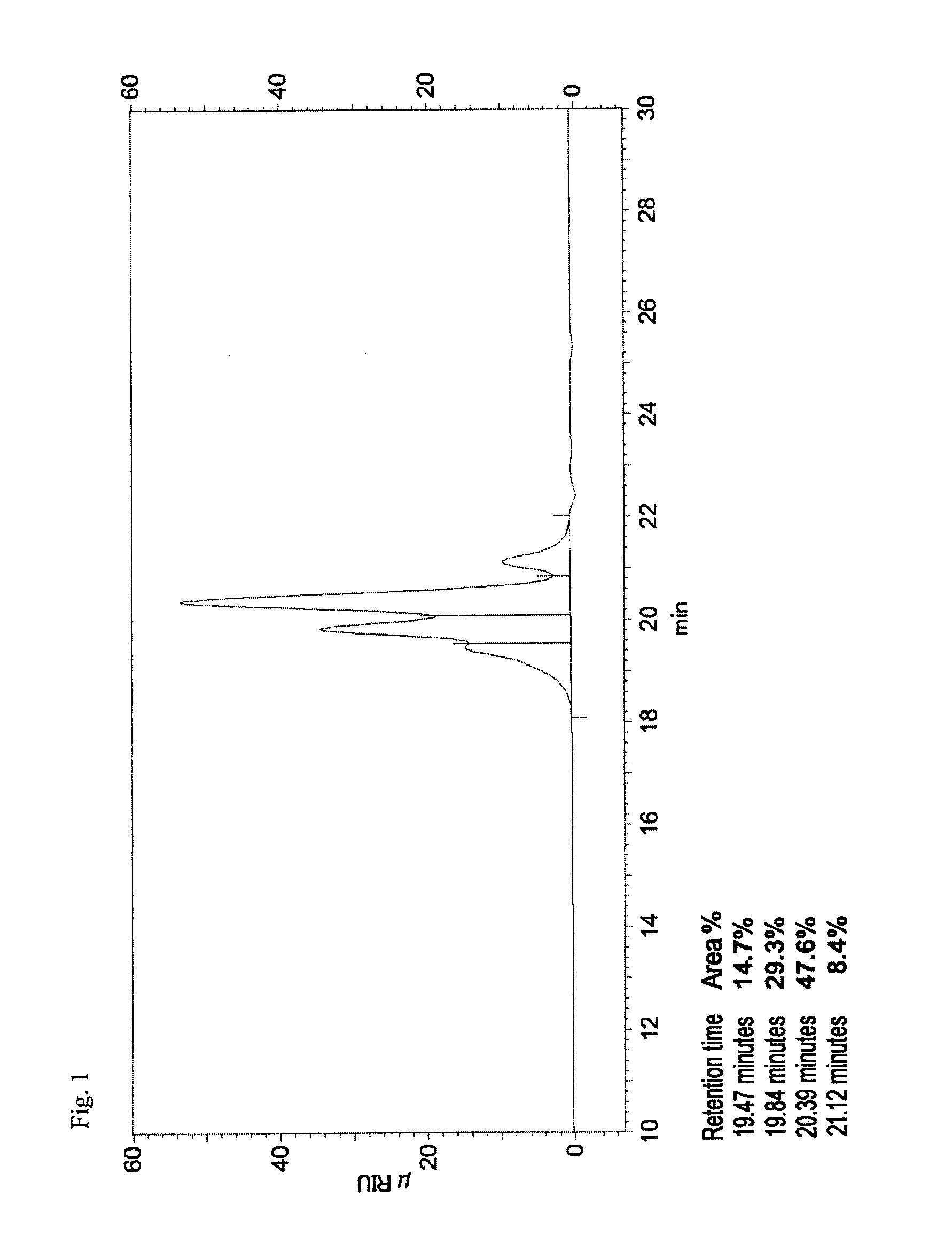
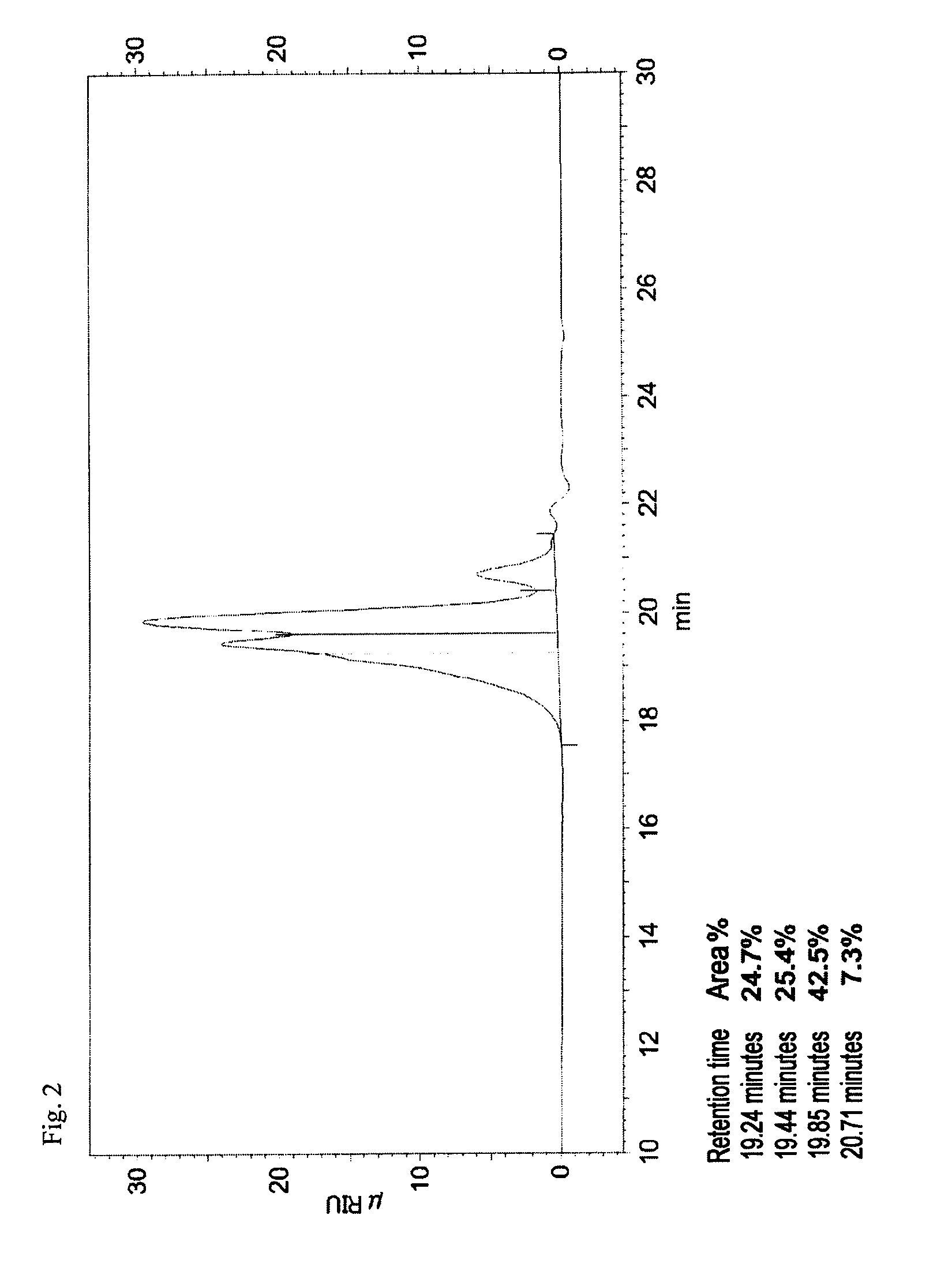
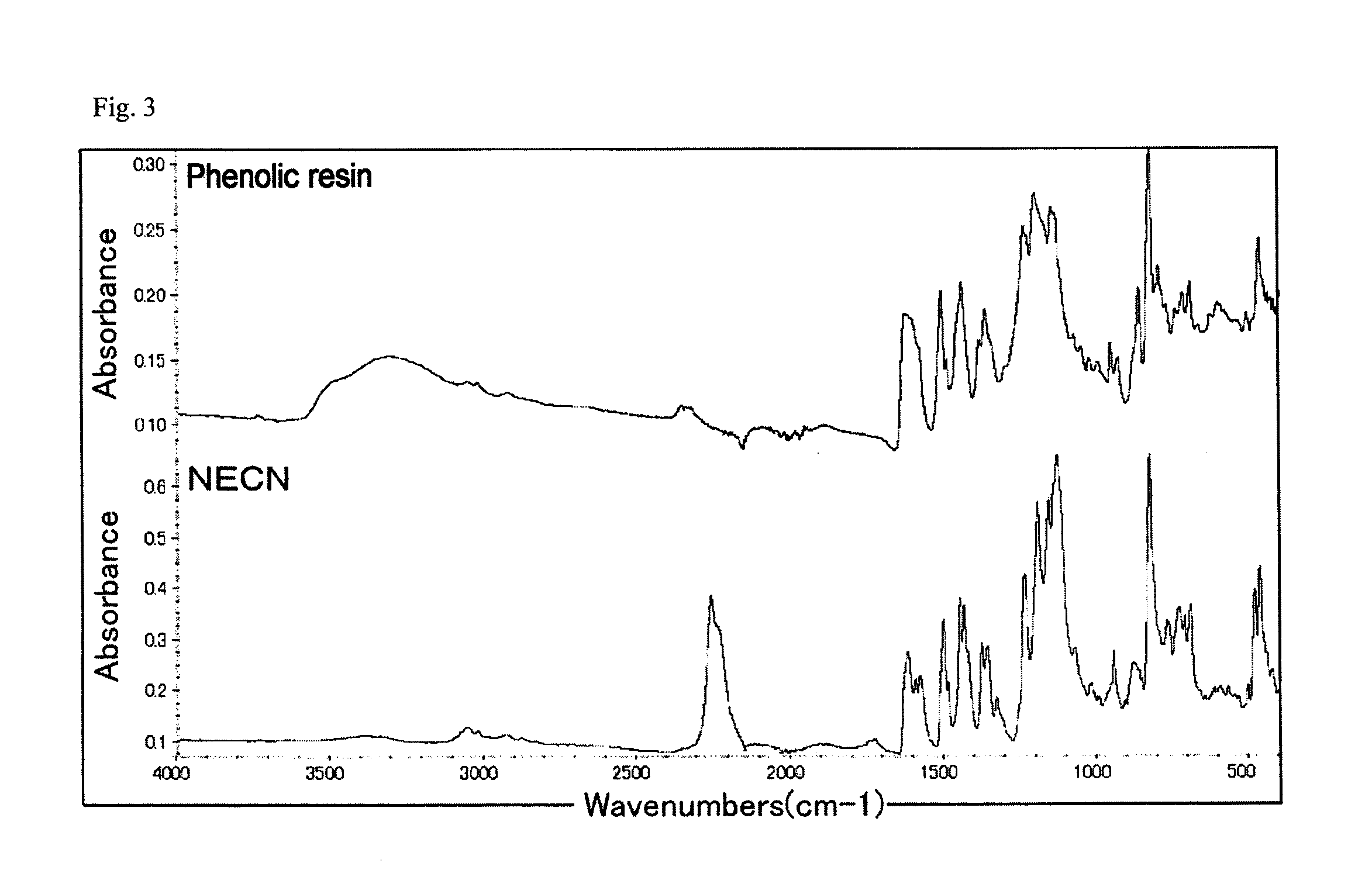
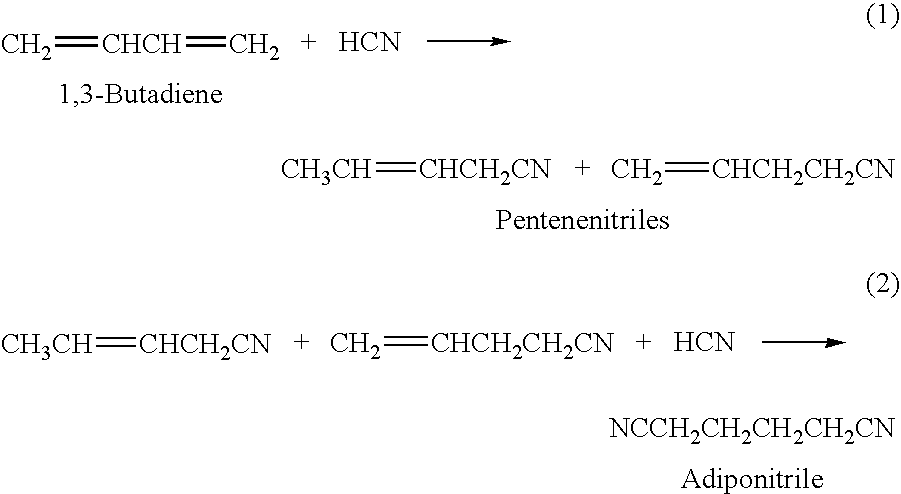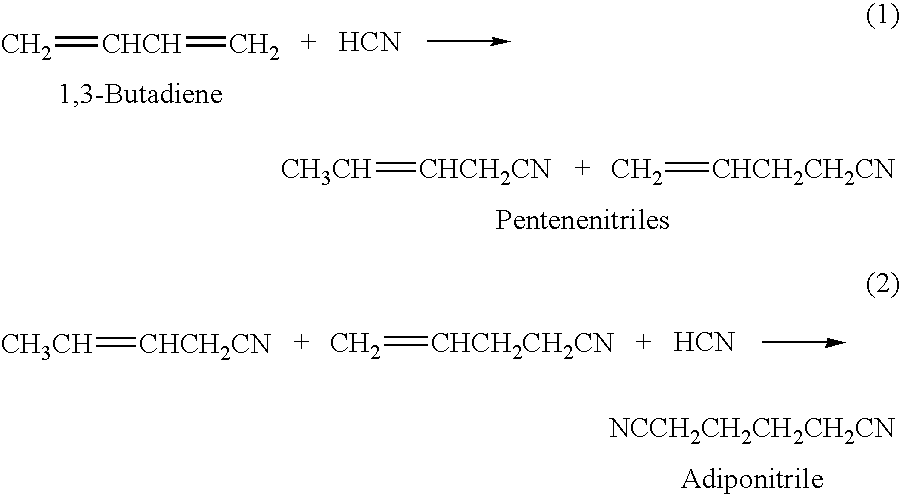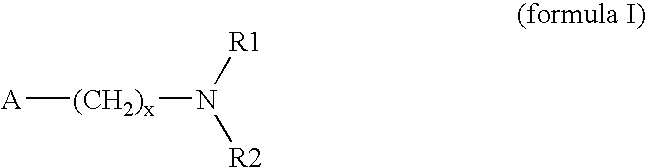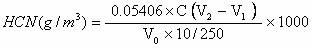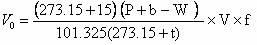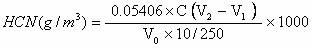Patents
Literature
161 results about "HCN poisoning" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydrogen cyanide (HCN), sometimes called prussic acid, is a chemical compound with the chemical formula HCN. It is a colorless, extremely poisonous and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F).
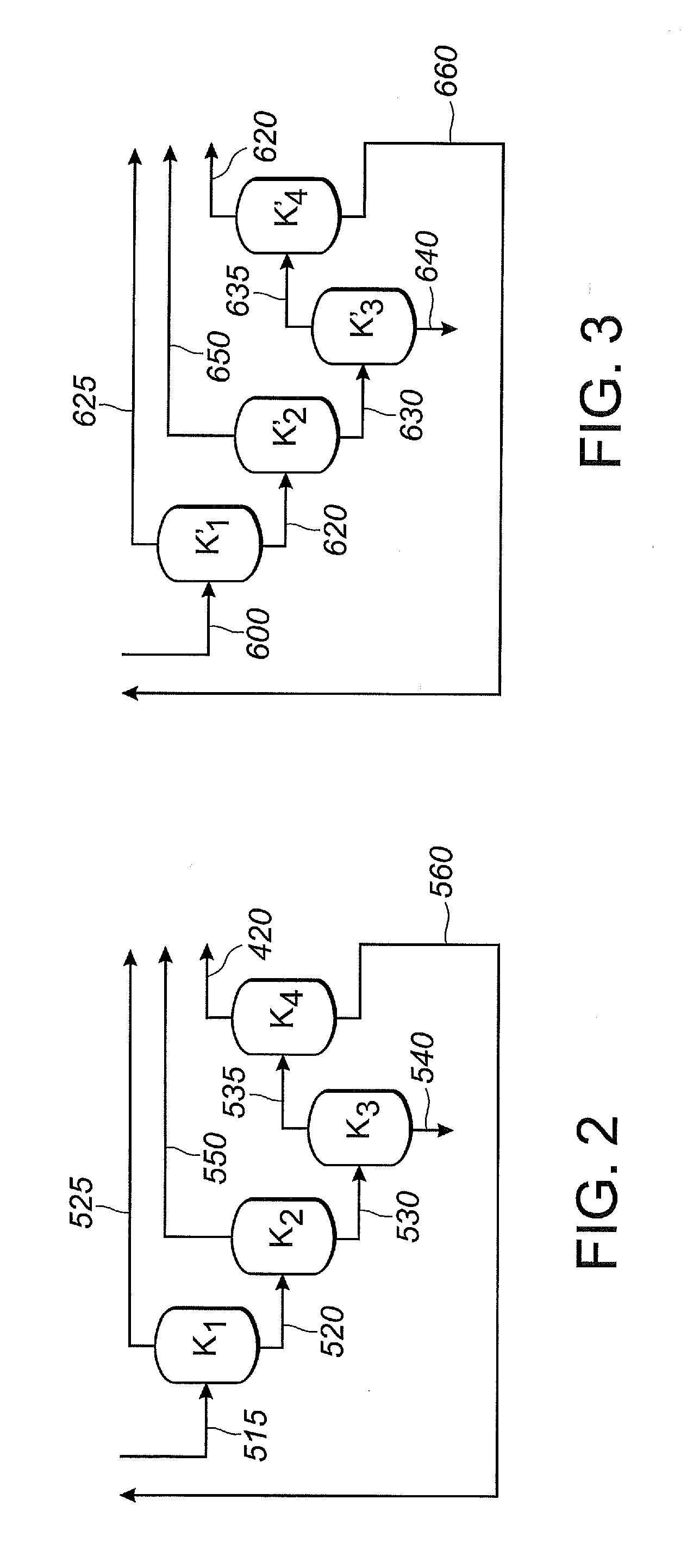
Etching method
InactiveUS20060226121A1Improves anisotropic propertyIncrease ratingsDecorative surface effectsSemiconductor/solid-state device manufacturingSimple Organic CompoundsHydrogen
An interlayer insulating film composed of an organic compound film containing an organic component as a main constituent is deposited on a semiconductor substrate. Then, etching is performed with respect to the interlayer insulating film by using a plasma derived from an etching gas containing an ammonia gas as a main constituent. As a result, active hydrogen is generated in the plasma derived from the ammonia gas to decompose the organic component into hydrogen cyanide, whereby etching proceeds. Since a surface of the organic compound film is efficiently nitrided by nitrogen generated from the ammonia gas, the sidewalls of a depressed portion in the organic compound film are protected so that an excellent anisotropic property is provided. Since the etching gas does not contain a component which oxidizes the organic compound film, the problem does not occur that a gas is generated from the organic compound film in a subsequent heat treatment process.
Owner:PANASONIC CORP
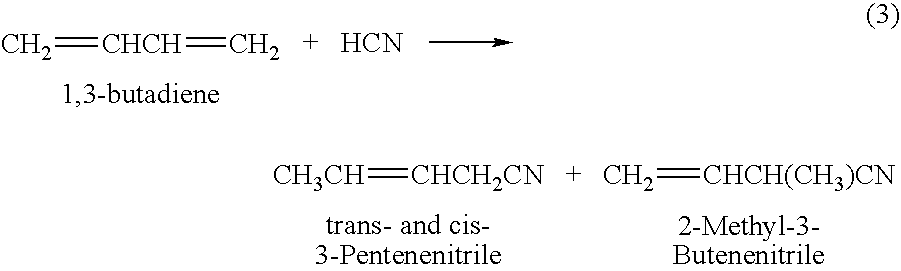
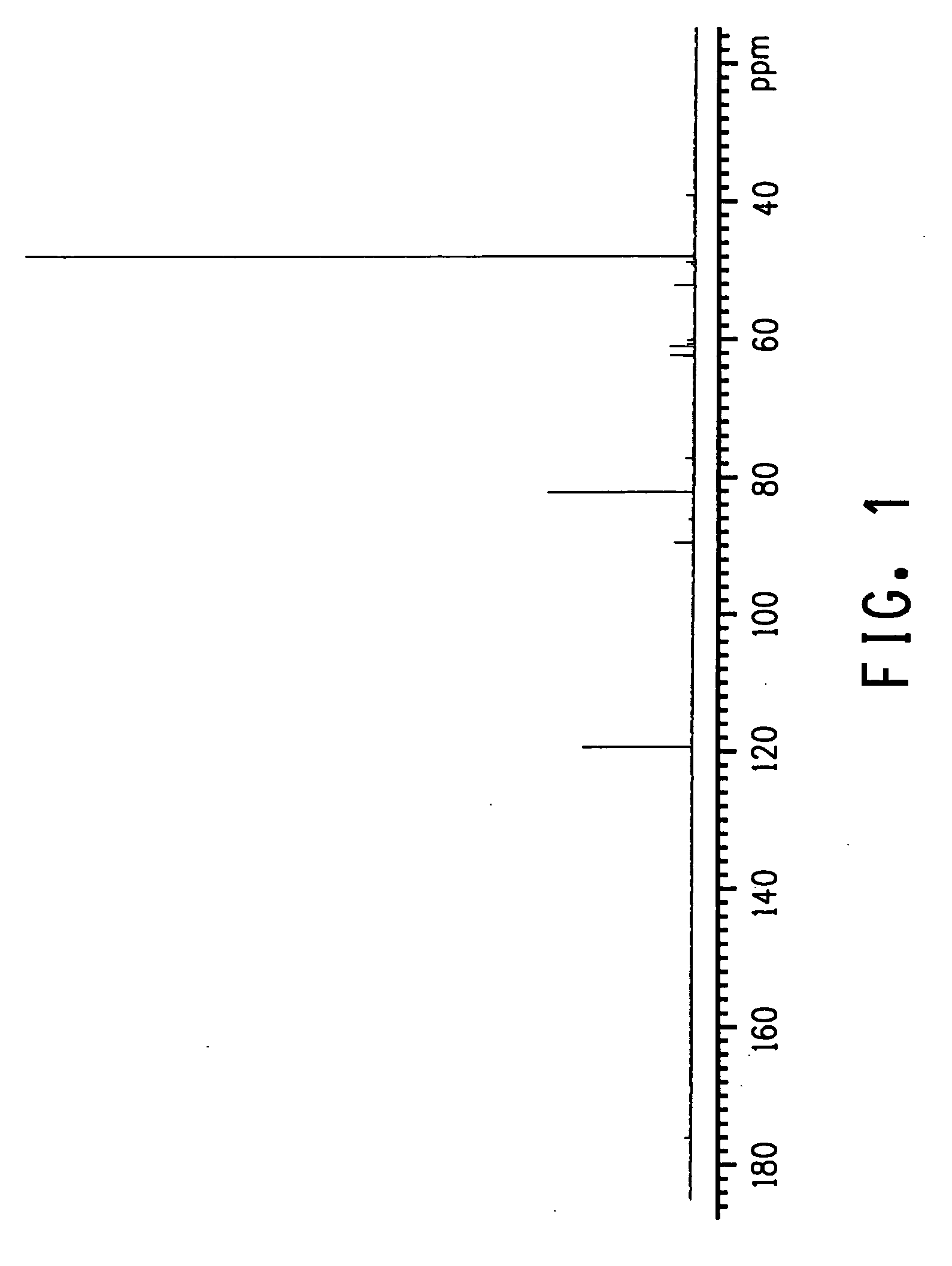
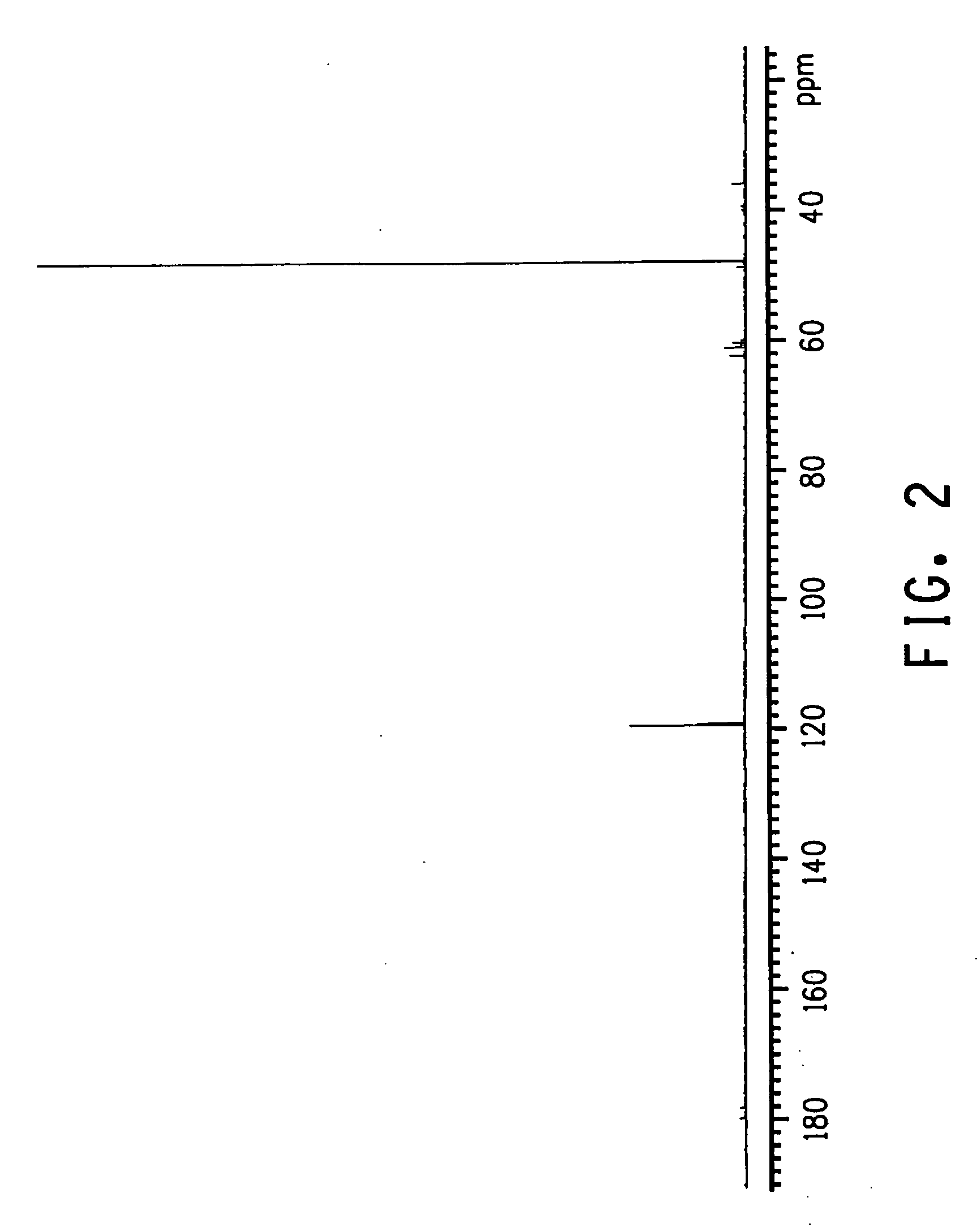
Method for producing 3-pentenenitrile
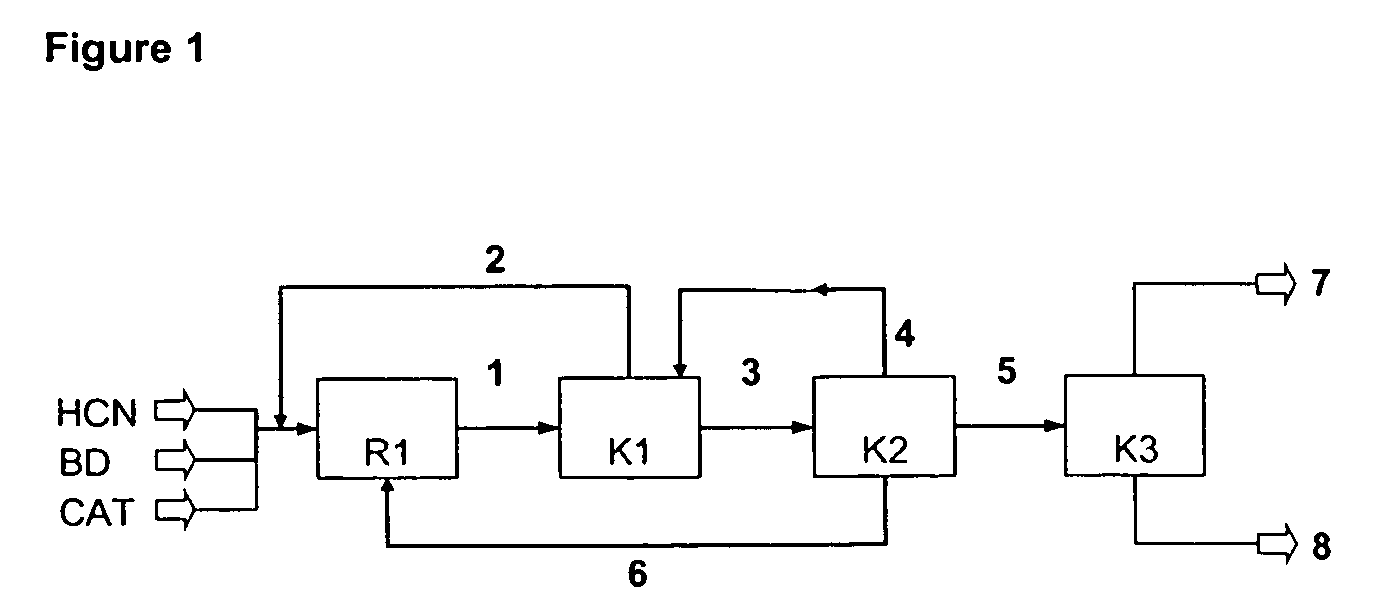
InactiveUS7541486B2Improve processing yieldReduce thermal stressOrganic compound preparationPreparation by hydrogen cyanide additionPtru catalystButadiene Dioxide
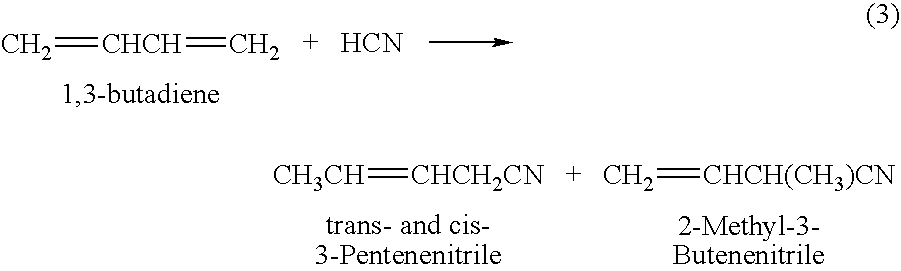
A process is described for preparing 3-pentenenitrile, characterized by the following process steps:(a) reacting 1,3-butadiene with hydrogen cyanide over at least one catalyst to obtain a stream 1 which comprises 3-pentenenitrile, 2-methyl-3-butenenitrile, the at least one catalyst and 1,3-butadiene,(b) distilling stream 1 in a column to obtain a high-1,3-butadiene stream 2 as the top product and a low-1,3-butadiene stream 3 as the bottom product which comprises 3-pentenenitrile, the at least one catalyst and 2-methyl-3-butenenitrile,(c) distilling stream 3 in a column to obtain a stream 4 as the top product which comprises 1,3-butadiene, a stream 5 which comprises 3-pentenenitrile and 2-methyl-3-butenenitrile at a side draw of the column, and a stream 6 as the bottom product which comprises the at least one catalyst,(d) distilling stream 5 to obtain a stream 7 as the top product which comprises 2-methyl-3-butenenitrile, and a stream 8 as the bottom product which comprises 3-pentenenitrile.
Owner:BASF AG
Hydrocyanation process with reduced yield losses
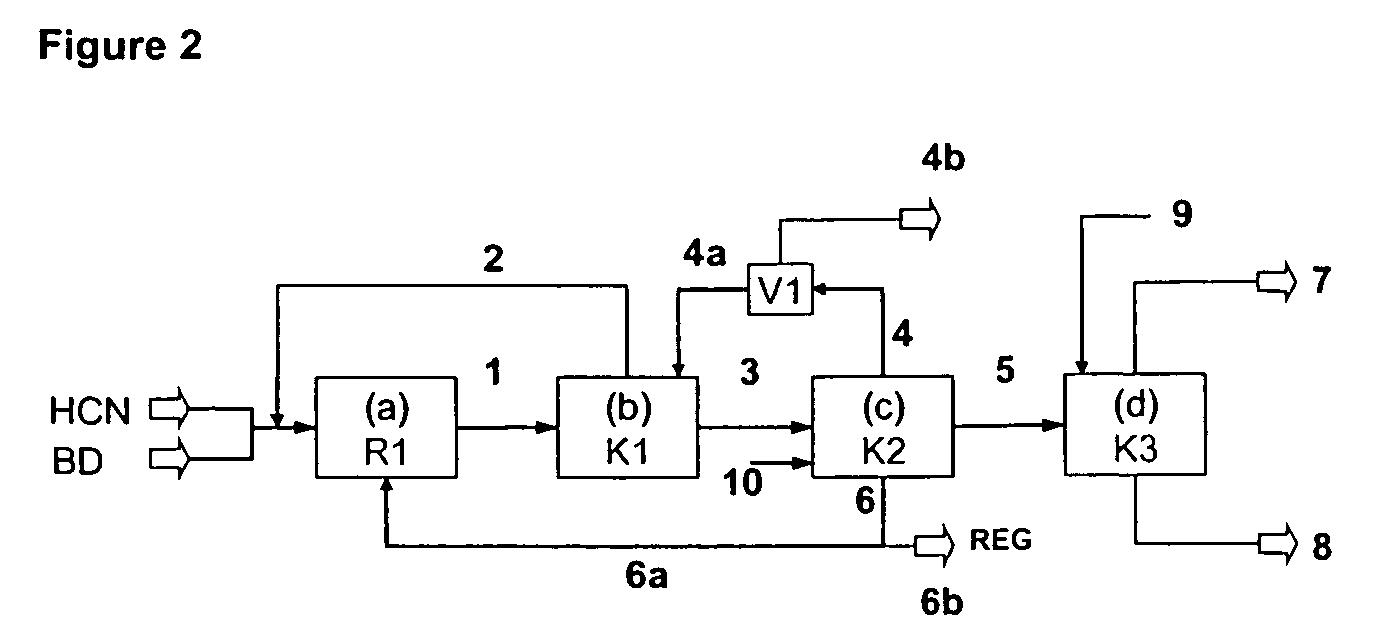
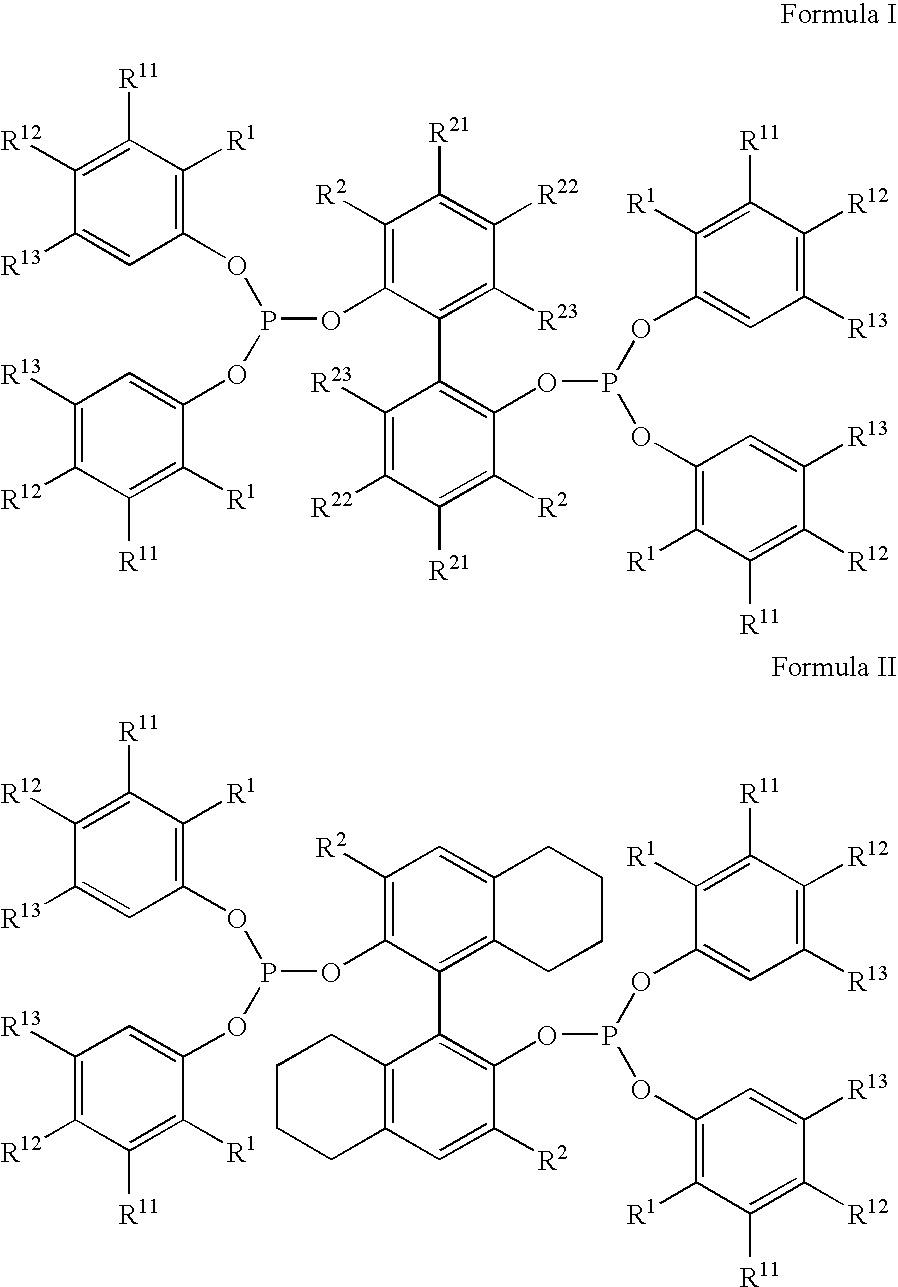
ActiveUS20080015381A1Investment exemptionSelective and efficient and stableOrganic compound preparationPreparation by hydrogen cyanide additionNitriteHydrogen
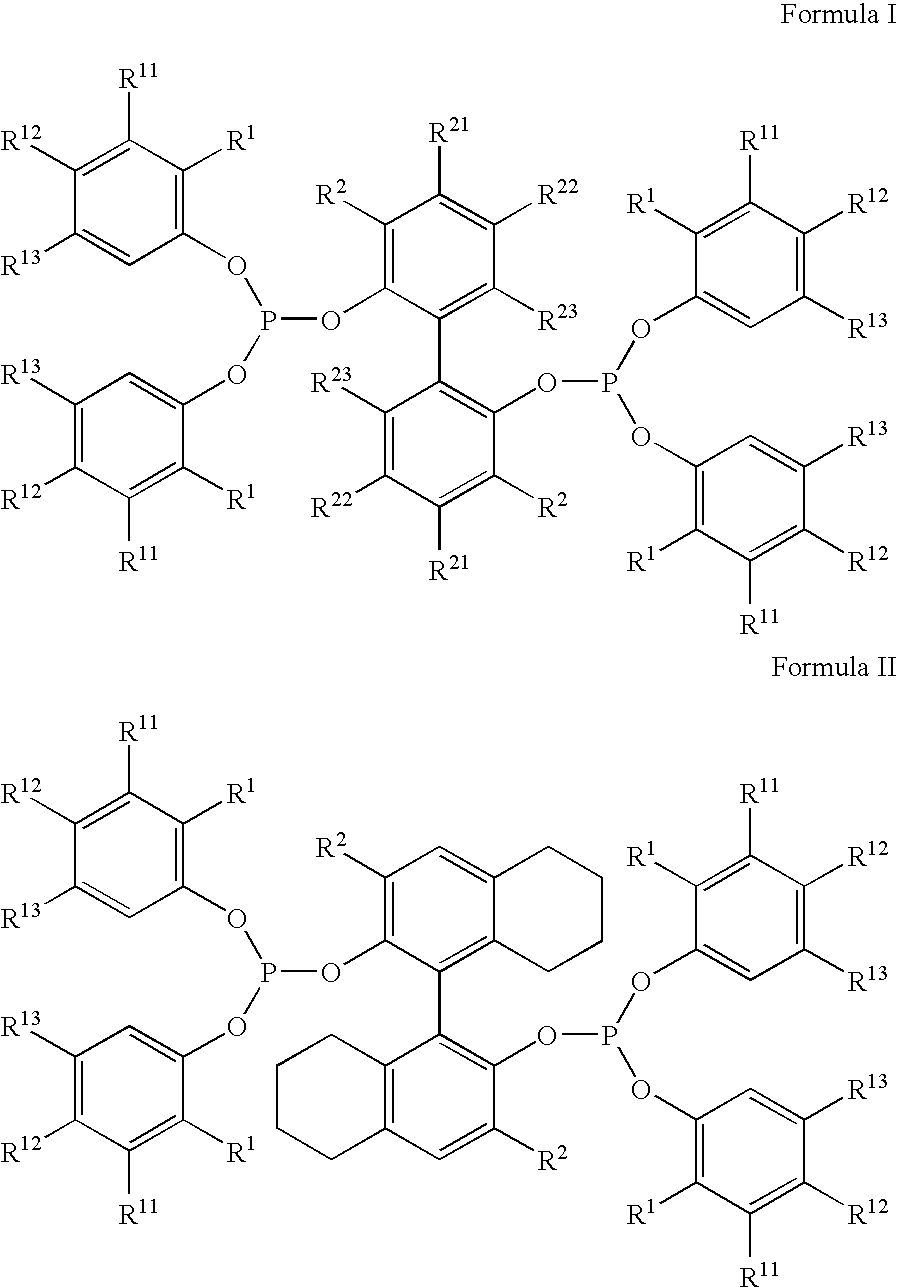
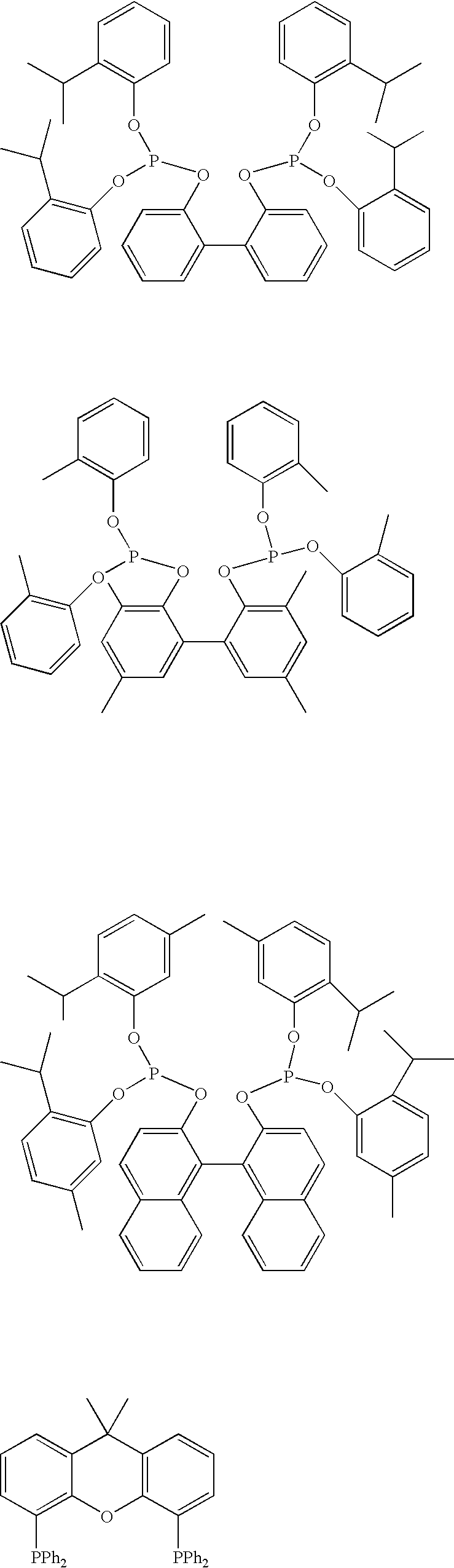
The invention provides a hydrocyanation process for the production of adiponitrile and other dinitriles having six carbon atoms, the process comprising:a) forming a reaction mixture in the presence of at least one Lewis acid, said reaction mixture comprising ethylenically unsaturated nitrites having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition, by continuously feeding ethylenically unsaturated nitrites, hydrogen cyanide, and a catalyst precursor composition;b) controlling X and Z, wherein X is the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and Z is the overall feed molar ratio of hydrogen cyanide to all unsaturated nitrites, by selecting a value for X in the range from about 0.001 to about 0.5, and a value for Z in the range from about 0.5 / 1 to about 0.99 / 1, such that the value of quotient Q, whereinQ=X[(moles3PN+4PNinthefeed)(molesallunsaturatednitrilesinthefeed)]-Zis in the range from about 0.2 to about 10, wherein 3PN is 3-pentenenitriles and 4PN is 4-pentenenitrile; andc) withdrawing a reaction product mixture comprising adiponitrile;wherein the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles in the reaction mixture is from about 0.2 / 1 to about 10 / 1;wherein the catalyst precursor composition comprises a zero-valent nickel and at least one bidentate phosphite ligand; andwherein the bidentate phosphite ligand is selected from a member of the group represented by Formulas I and 11 as described herein.
Owner:INV NYLON CHEM AMERICAS LLC
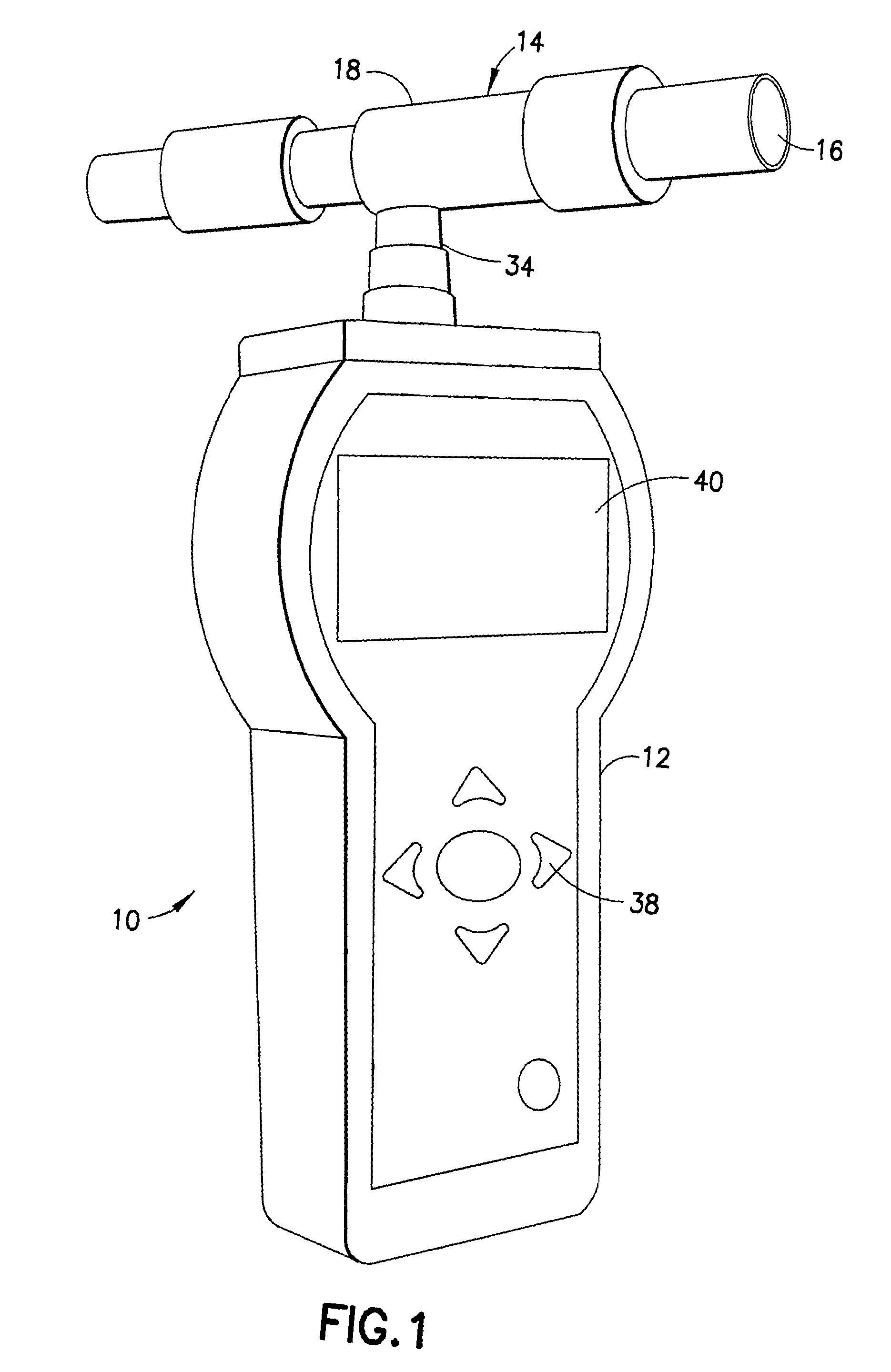
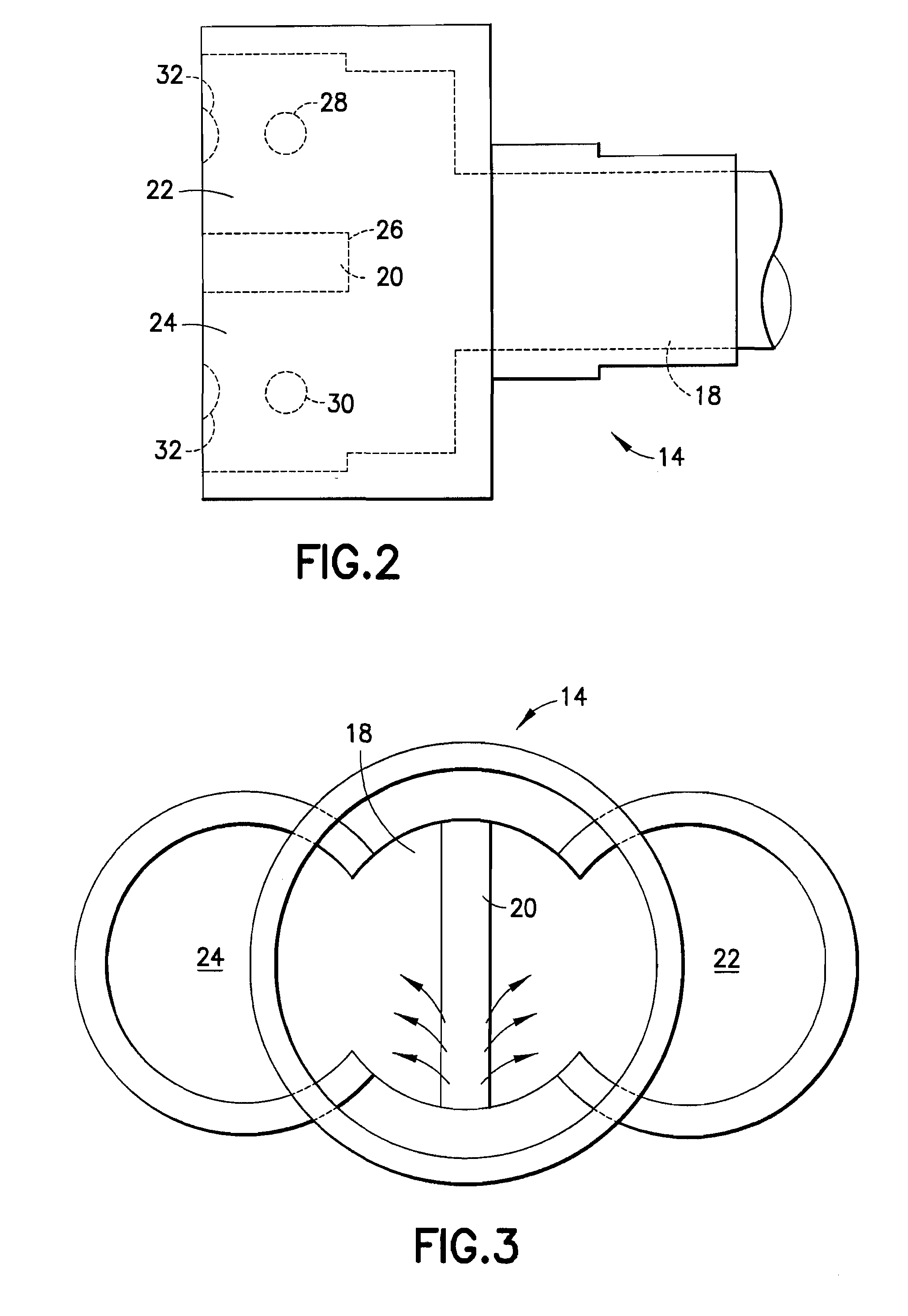
Gas analyzer
InactiveUS20090187111A1Quickly determineQuick levelingWithdrawing sample devicesRespiratory organ evaluationToxic gasHydrogen
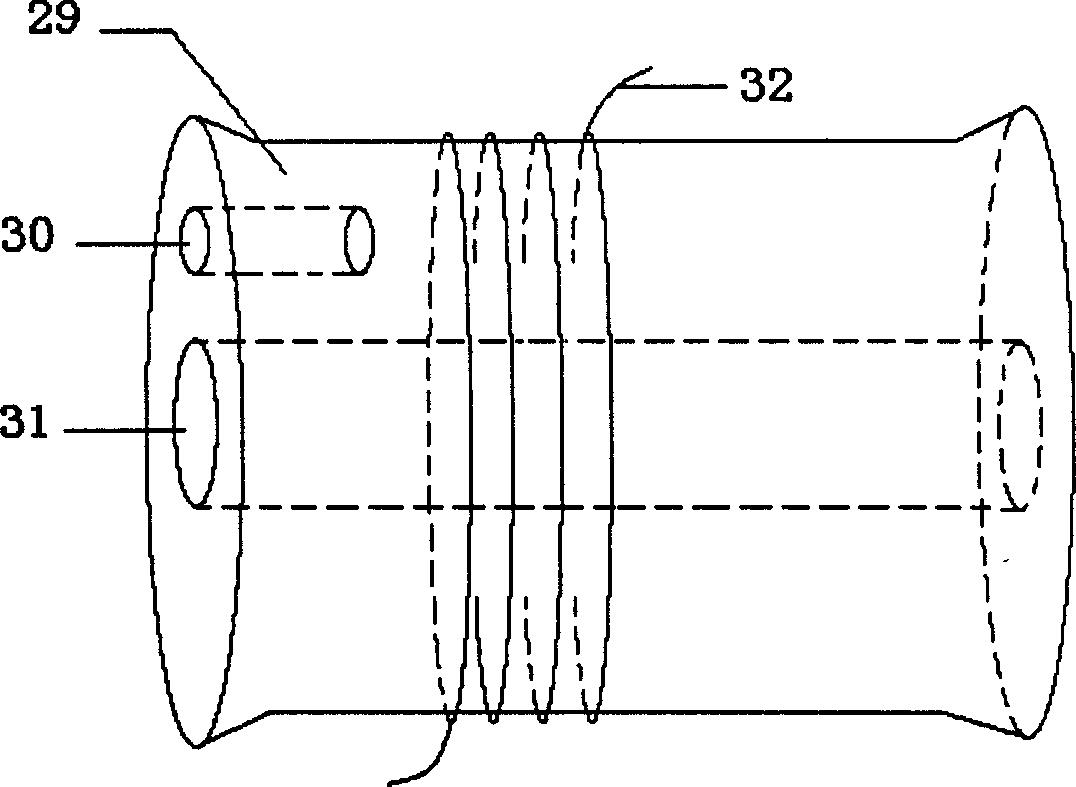
The subject invention is directed to a breath analyzer which is capable of detecting toxic gas levels from breath analysis. The subject invention includes a mouthpiece which is in communication with a plurality of discrete chambers, such as first and second discrete chambers, each being provided with a separate probe for breath analysis. The probes are connected to analyzers for determining detected levels of gas. In a first embodiment, a first probe may be provided for carbon monoxide detection with a second probe being provided for hydrogen cyanide detection. Advantageously, with this arrangement, breath analysis may be conducted on-site, for example at the site of a fire, to quickly and simultaneously determine carbon monoxide and hydrogen cyanide levels in a person's blood stream. In a second embodiment, a first probe may be provided for detection of carbon monoxide and a second probe may be provided for detection of hydrogen. With this arrangement, a calibrated correction of measured carbon monoxide data can be made to correct for improperly detected hydrogen. As such, a highly accurate on-site measurement for carbon monoxide can be achieved.
Owner:FSP INSTR
Hydrocyanation process with reduced yield losses
ActiveUS7659422B2Investment exemptionSelective and efficient and stableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenHydrogen cyanide
A hydrocyanation process produces adiponitrile and other dinitriles having six carbon atoms. The process involves forming a reaction mixture in the presence of at least one Lewis acid. The reaction mixture includes ethylenically unsaturated nitriles having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition. The reaction mixture is continuously fed while controlling the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and the overall feed molar ratio of hydrogen cyanide to all unsaturated nitriles. In the reaction product mixture, including adiponitrile, the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles is from about 0.2 / 1 to about 10 / 1. Included in the catalyst precursor composition is a zero-valent nickel and at least one bidentate phosphite ligand.
Owner:INV NYLON CHEM AMERICAS LLC
Process for the synthesis of glycolonitrile
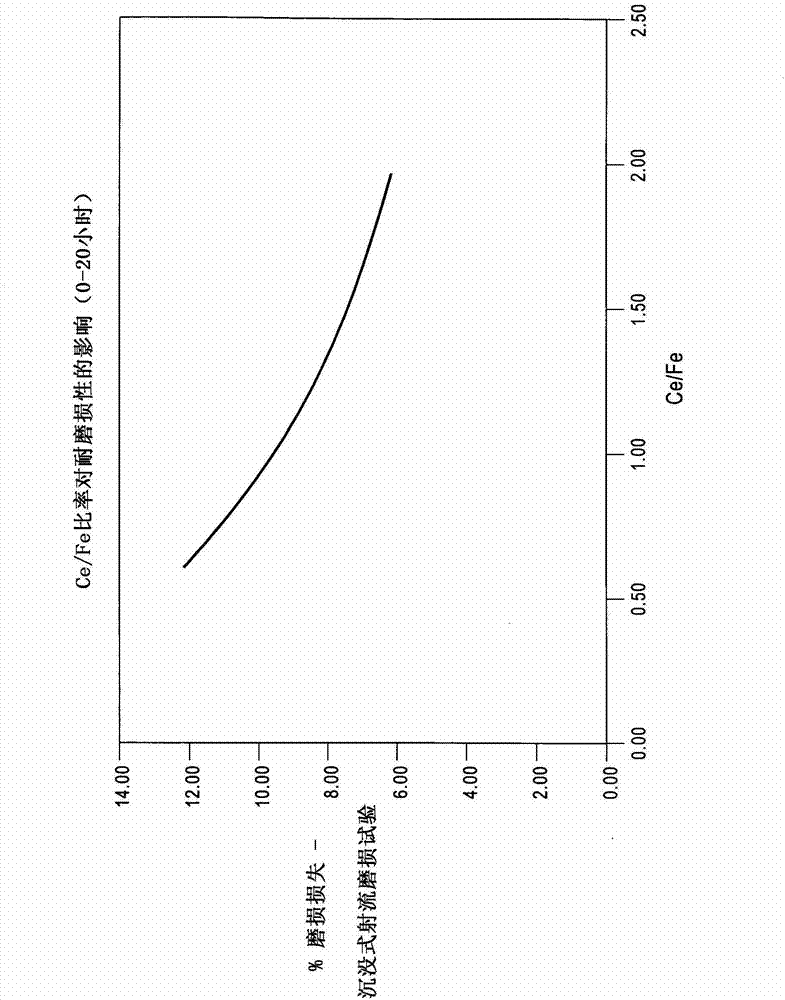
ActiveUS20060160196A1Minimize glycolonitrile decompositionOrganic compound preparationPhosphorus organic compoundsGlycollic acidHCN poisoning
A process to prepare substantially pure glycolonitrile in an aqueous medium is provided by reacting hydrogen cyanide and formaldehyde. The formaldehyde feed stream is heated prior to reacting with hydrogen cyanide, resulting in an aqueous glycolonitrile solution with fewer impurities, especially less unreacted formaldehyde, than is obtained by other methods. The process enables production of an aqueous glycolonitrile solution that requires less post-reaction purification (if any at all) prior to enzymatically converting the glycolonitrile into glycolic acid.
Owner:THE CHEMOURS CO FC LLC
Process of synthesis of compounds having nitrile functions from ethylenically unsaturated compounds
InactiveUS20060252955A1High selectivityReduce instabilityOrganic compound preparationPreparation by hydrogen cyanide additionIsomerizationHCN poisoning
A process of hydrocyanation of diolefins such as butadiene is carried out in the presence of a catalytic system comprising a transition metal and mono- and pluri-dentate organophosphorus ligands. The reaction medium containing branched nitrites subsequently is isomerized in the absence of hydrogen cyanide.
Owner:INV NYLON CHEM AMERICAS LLC
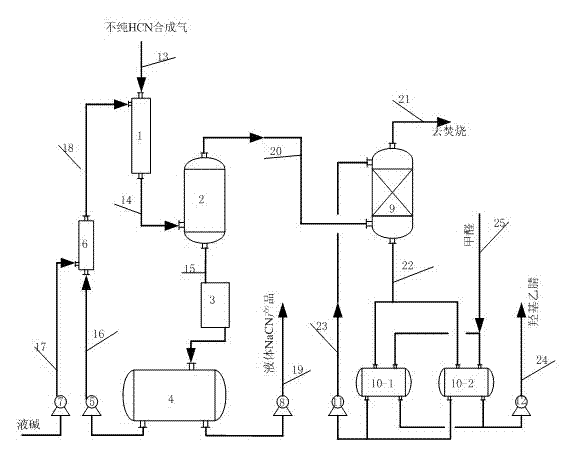
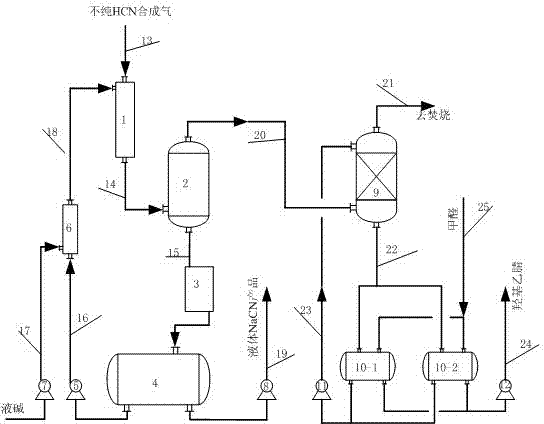
Preparation method for preparing alkali metal or alkali earth metal cyanide with high purity and high yield
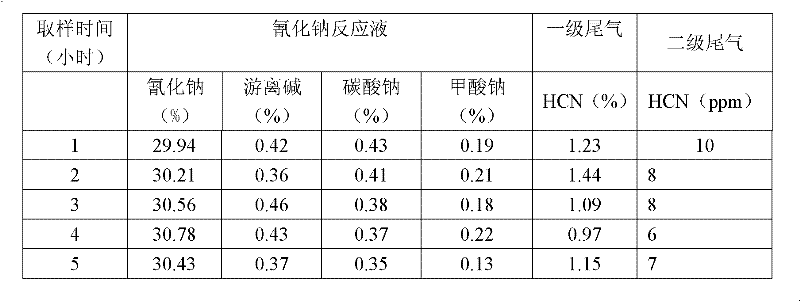
ActiveCN102502708AHigh purityGood colorAlkali metal cyanidesChemical industryCyanideAlkaline earth metal
The invention relates to a preparation method for preparing alkali metal or alkali earth metal cyanide with high purity and high yield, which comprises the following steps of: mixing, absorbing and reacting HCN (hydrogen cyanide) synthetic gas synthesized by an Andrussow method with absorption liquid containing alkali and then carrying out gas-liquid separation and cooling to prepare the alkali metal or the alkali earth metal cyanide. The absorption and reaction contact time of the HCN synthetic gas with the absorption liquid containing the alkali can be controlled within 0.1-2.0 seconds; the concentration of free alkali in the absorption liquid containing the alkali can be controlled within 0.1-20 percent by weight; and the mass ratio of circulation fluid of the cyanide to product liquid is 2-40:1. The invention has the beneficial effects that the alkali metal or the alkali earth metal cyanide prepared by the preparation method is high in purity; the total content of carbonate can be controlled within below 0.45 percent by weight, the content of formate can be controlled within below 0.15 percent by weight, and the content of ocyhydrate (free alkali) can be controlled within 0.1-2.0 percent by weight; and the alkali metal or the alkali earth metal cyanide is good in color and is colorless transparent liquid.
Owner:NINGXIA UNISPLENDOUR TIANHUA METHIONINE CO LTD
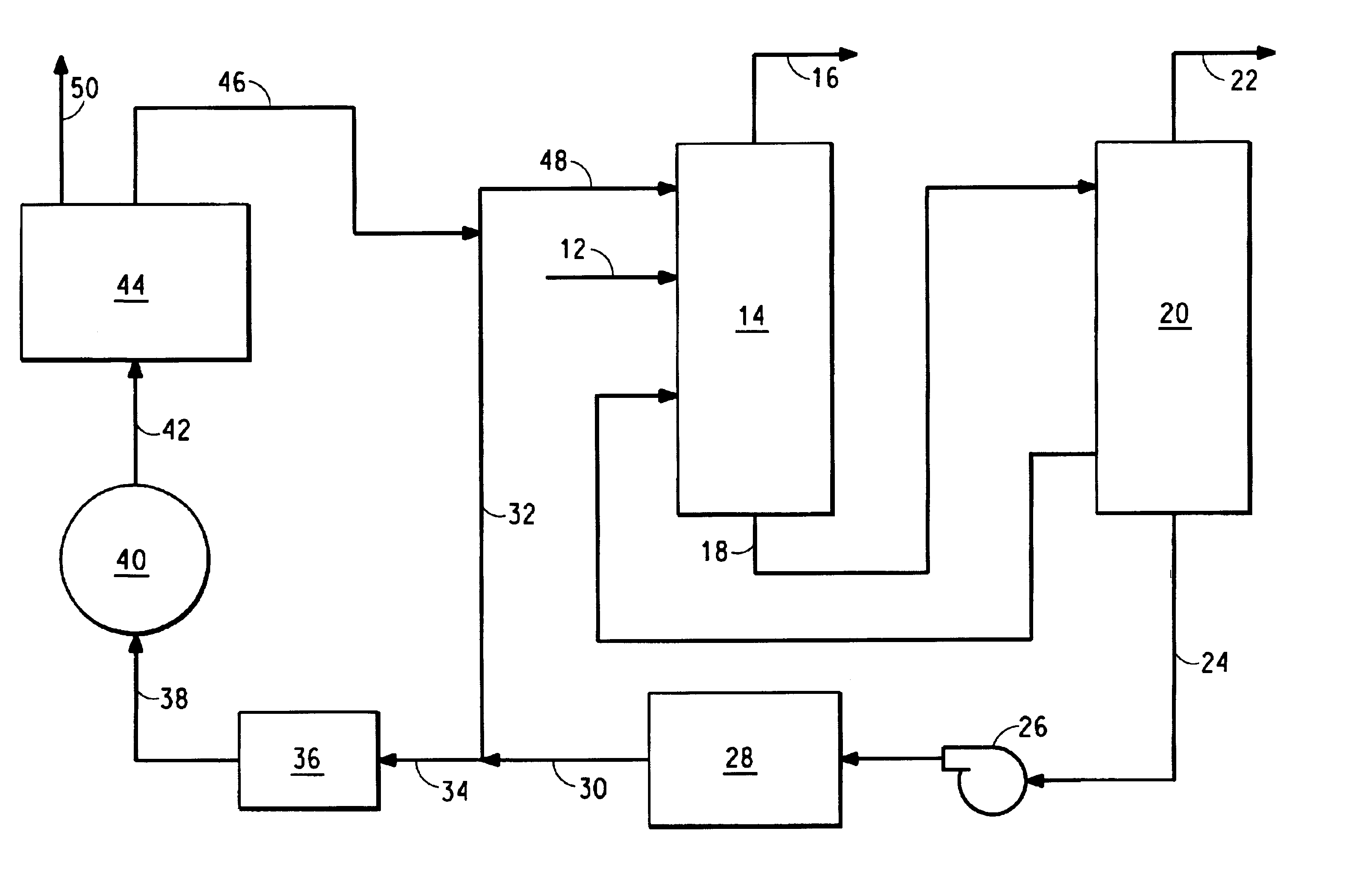
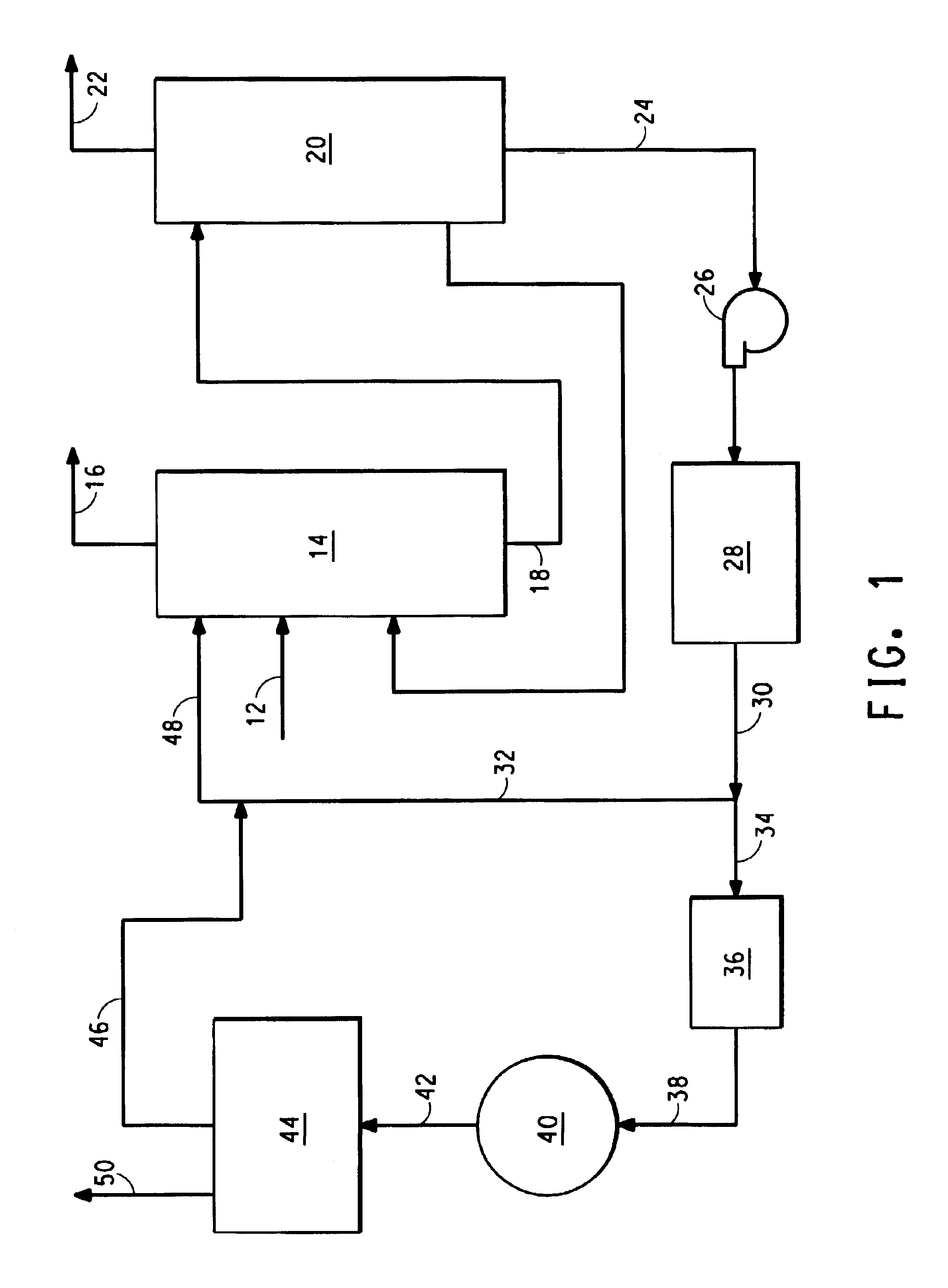
Method for removing ammonia from a gas comprising hydrogen cyanide, ammonia and water
InactiveUS6872296B2Low cost of treatmentReduce waste generationPolycrystalline material growthLiquid degasificationChemistryCarbon dioxide
A method for removing ammonia from a gas comprising HCN, ammonia and water, by first contacting the gas with aqueous ammonium phosphate to remove ammonia from the gas, and then passing the resulting ammonium phosphate solution through an electrolyzer to convert oxalates and formates into hydrogen and carbon dioxide.
Owner:INVISTA NORTH AMERICA R L
Method for preparing 3-(methylthio) propanal
ActiveUS20060030739A1High selectivityMinimizing high-boiling by-productsCarboxylic acid nitrile preparationOrganic compound preparationAcroleinHCN poisoning
Owner:EVONIK DEGUSSA GMBH
Treatment method and system of cyanogens-containing organic waste gas
PendingCN107051144APromote absorptionDecompose thoroughlyGas treatmentDispersed particle separationChlorine dioxideAcid washing
The invention discloses a treatment method of cyanogens-containing organic waste gas. The waste gas is subjected to gas collection, is firstly treated by a cooler and a defoaming device, and then enters an acid washing tower to be subjected to acid washing and spraying so that ammonia gas and partial organic matters in the waste gas can be removed; then, the waste gas enters an alkaline washing tower and is sprayed by alkaline liquid; hydrogen cyanide and alkaline liquid take a reaction to produce a sodium cyanide water solution; partial water-soluble organic matters are absorbed by the alkaline liquid; the low-concentration organic waste gas discharged from the alkaline washing tower enters a catalytic oxidization tower, is absorbed by spraying liquid containing oxidizing agents of chlorine dioxide sprayed from the tower top, and then takes an oxygenolysis reaction through a catalyst bed layer; the rest waste gas is exhausted in a standard reaching way after finally passing an active carbon absorption tower. In the treatment process, no waste water or only a small amount of waste water is generated; the HCN in the waste gas is oxidized into harmless nontoxic and harmless N2 and CO2; the HCN removal rate can reach 99 percent or higher, so that the cyanide in the waste liquid is discharged in a standard reaching manner; the whole waste gas treatment process is simple; the treatment effect is good.
Owner:ZHEJIANG QICAI ECO TECH CO LTD
Process for making nitriles
ActiveUS20130150610A1Minimize consequencesOrganic compound preparationChemical recyclingHydrogen cyanideHydrocyanation
A hydrocyanation reaction is used to react 1,3-butadiene with hydrogen cyanide in the presence of a catalyst to produce pentenenitriles, as well as reaction byproducts, such as methylglutaronitrile (MGN). The effluent from the hydrocyanation reaction is distilled in a particular manner to produce a pentenenitrile-enriched stream, a catalyst-enriched stream and a stream enriched in methylglutaronitrile (MGN). At least a portion of the catalyst enriched stream may be recycled to the hydrocyanation reaction. 3-pentenenitrile may be recovered and, optionally, further reacted with HCN to make adiponitrile (ADN).
Owner:INV NYLON CHEM AMERICAS LLC
Flow injection colorimetric cyanogens detection device and cyanogens detection method thereof
InactiveCN1699976AHigh degree of automationFast measurementMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsCyanide compoundPeristaltic pump
The invention relates to an apparatus and method for measuring the content of cyanide, the said apparatus comprises front treating apparatus and color measuring apparatus, wherein front treating apparatus includes peristaltic pump, automatic sampling device and online heating module which are connected with peristaltic pump; the said online heating module connects with online distilling module and six relaxing valve of coloring and measuring apparatus, wherein online distilling module is connected with sodium hydroxide absorbing liquid container linked with peristaltic pump; the said three relaxing pipe is connected with pipe with distilling regent solution. The method includes preparing miscellaneous needed solution and color measuring in the medium of pH3-4; distilling cyanides as hydrogen cyanides by online distilling; separating other disturbance by gas permeable membrane; inputting regent solution into hydrogen cyanic acid; doing colorimetric measuring by optical fiber spectrometer at wavelength of 580nm.
Owner:BEIJING JITIAN INSTR CO LTD
Process for producing high-quality acrylamide polymer with enzyme
The object of the present invention is to obtain a highly soluble and colorless acrylamide polymer having a high molecular weight. A method for producing such acrylamide polymer involves comprises steps of: hydrating acrylonitrile containing oxazole at a concentration of 5 mg / kg or less and hydrogen cyanide at a concentration of 1 mg / kg or less by an enzymatic method to yield acrylamide; andt polymerizing monomers containing acrylamide.
Owner:DAIYANITORIKKUSU KK
Method for testing content of hydrogen cyanide in gas
InactiveCN102636616ARapid determinationAccurate measurementChemical analysis using titrationPotassium hydroxideSulfide
The invention provides a method for testing content of hydrogen cyanide in gas. The method comprises the following steps of: absorbing the hydrogen cyanide in the gas by adopting a potassium hydroxide solution, transferring the absorption solution to a volumetric flask with a certain volume, taking a certain amount of absorption solution, adding a cadmium acetate solution to make sulfide in the absorption solution to form indissolvable cadmium sulfide to sediment, filtering and sintering the absorption solution to eliminate the sediments, taking Dabr as an indicator of the solution, taking a standard silver nitrate solution to titrate, making cyanide ions react with silver nitrateto form soluble cyanide complex ion (Ag(CN)2), making excessive silver ions react with the Dabar indicator so as to make the color of the solution change from yellow to orange to the end, and calculating the content of the hydrogen cyanide according to the volume of the consumed standard silver nitrate solution. The method provided by the invention solves the problems that various kinds of reagents used in the prior art are poisonous and harmful, and moreover the reagents have the disadvantages of complex analysis step, long elapsed time and the like, have potential safety hazards existing in the testing process, and enhance the sewage treatment load of the back section. The method can rapidly and accurately test the content of the hydrogen cyanide in coke oven coal.
Owner:云南昆钢煤焦化有限公司
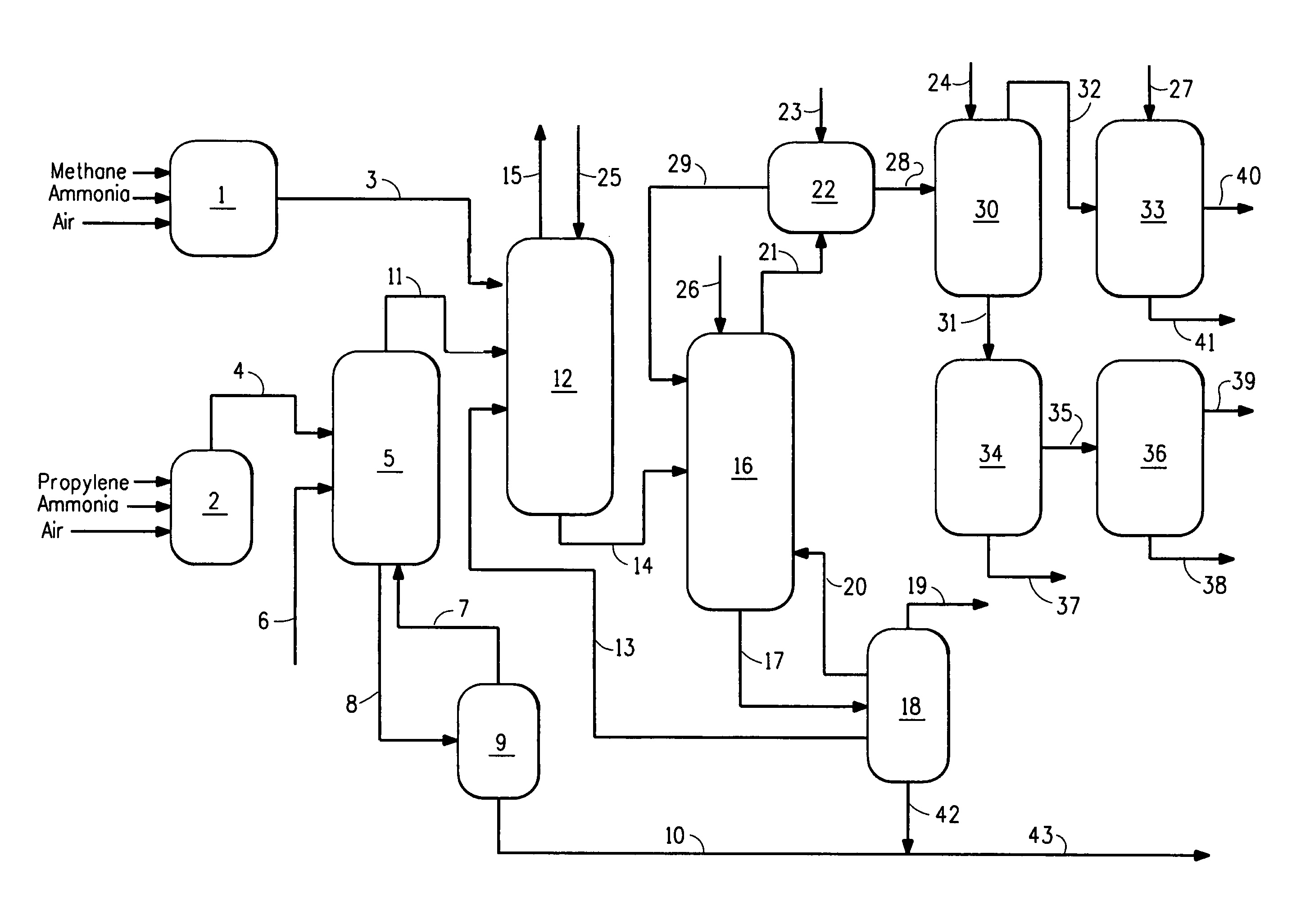
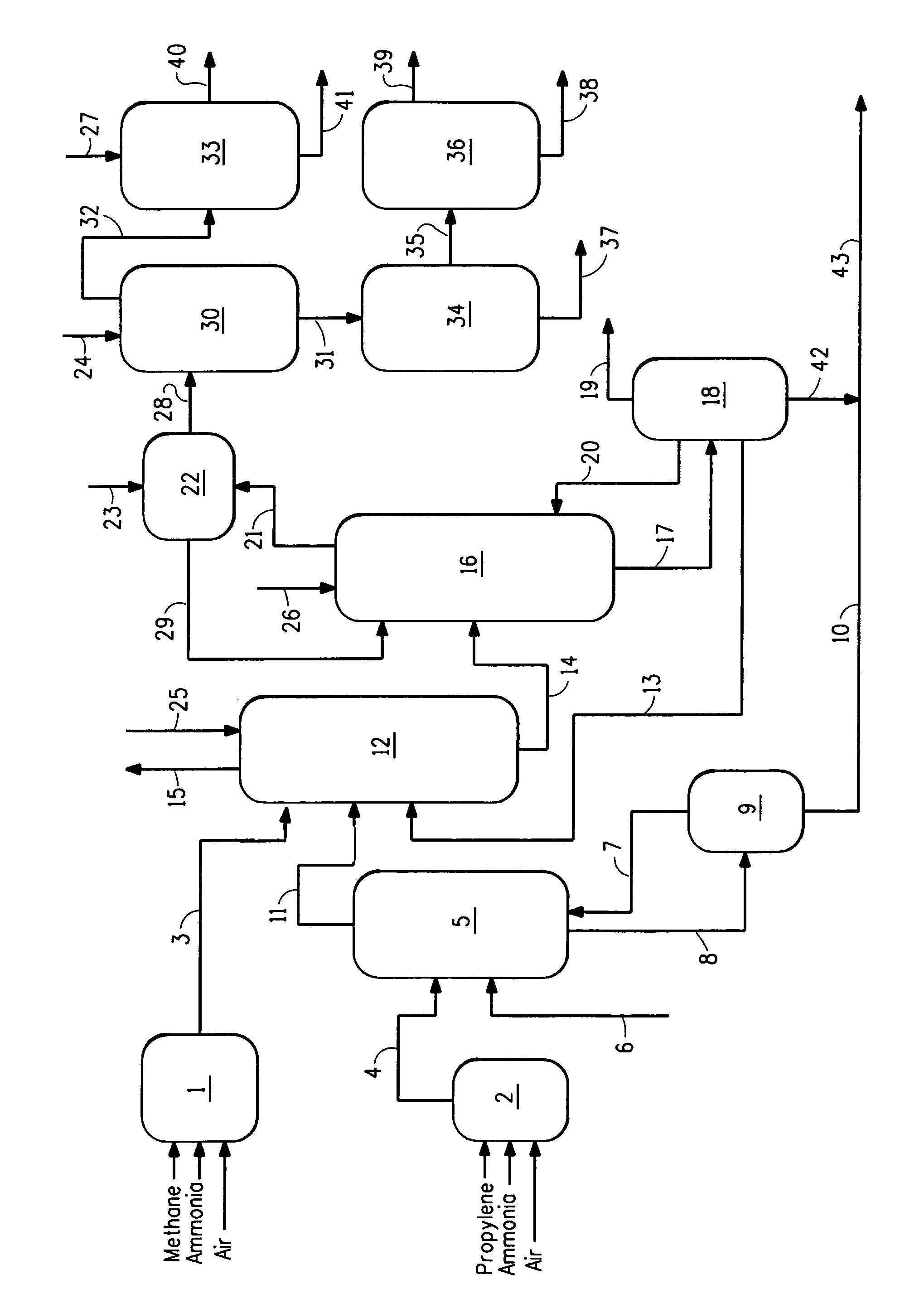
Process to C-manufacture acrylonitrile and hydrogen cyanide
ActiveUS8585870B2Conveniently providedWeight controlOrganic compound preparationUsing liquid separation agentAcrylonitrilePhotochemistry
A process for co-manufacture of acrylonitrile and hydrogen cyanide comprises combining a stream comprising hydrogen cyanide and an acrylonitrile reactor product stream, to produce a combined product stream, having a ratio of acrylonitrile to hydrogen cyanide of about 9 to 1 or less, which can be varied; and treating the combined product stream in a recovery / purification system of acrylonitrile process wherein pH is controlled by addition of an acid to prevent HCN polymerization. The ratio of acrylonitrile to hydrogen cyanide is generally between 2 to 1 and 9 to 1. The stream comprising hydrogen cyanide is advantageously a hydrogen cyanide product stream from a hydrogen cyanide synthesis reactor.
Owner:THE CHEMOURS CO FC LLC
Pre calcination additives for mixed metal oxide ammoxidation catalysts
ActiveUS8835666B2High productImprove utilization efficiencyUrea derivatives preparationOrganic compound preparationGas phaseNitrogen
A process for preparation of catalysts for the production of acrylonitrile, acetonitrile and hydrogen cyanide comprising contacting at an elevated temperature, propylene, ammonia and oxygen in the vapor phase in the presence of a catalyst, said catalyst comprising a complex of metal oxides wherein a heat-decomposable nitrogen containing compound is added during the process for the preparation of the catalyst.
Owner:INEOS EURO LTD
A kind of method that catalyzes hydrocyanic acid and oxirane to synthesize 3-hydroxy propionitrile
ActiveCN106883142BSubtractive synthesis processReduce operational riskOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by cyanide reactionInorganic saltsCatalytic method
The complex of boron trifluoride and 1,2-pentanediol immobilized on activated alumina is used to catalyze the reaction of hydrogen cyanide and ethylene oxide to synthesize 3-hydroxypropionitrile. Utilizing this method, there is no need for a large amount of acid to neutralize the reaction mixture during the reaction, and a large amount of inorganic salts are not produced. It is a reaction with 100% atom economy. It has a short reaction route, few operating steps, and easy product separation, making it a simple and easy-to-operate reaction. ; Under low-temperature reaction conditions, there is no risk of large amounts of hydrogen cyanide overflowing in the system, and it is a safe reaction to operate.
Owner:FUSHUN SHUNTE CHEM
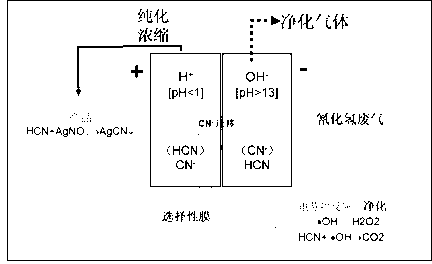
Method and device for purifying hydrogen cyanide industrial waste gas by electrodialysis
ActiveCN103071370AHigh selectivityIncrease added valueDispersed particle separationCyanide ionCobalt
The invention discloses a method and a device for purifying hydrogen cyanide industrial waste gas by electrodialysis. The method comprises the steps as follows: filling the hydrogen cyanide waste gas into a cathode, and purifying the hydrogen cyanide waste gas by utilizing free radicals with strong oxidizing property generated by electro-fenton reaction at the cathode; purifying and concentrating part of cyanide ions which are not fully oxidized by utilizing the selective permeability of a cobalt-containing perfluoro-sulfonate ion exchange membrane at an anode; and adding hydrocyanic acid purified and concentrated at the anode with different types of salts to prepare cyanide which is low in toxicity and easy to store and transport. According to the method, the liquid phase adsorption capacity is increased, the harmful gas purifying capacity can be increased by utilizing the liquid phase adsorption, electrodialysis and ion exchange principles while higher gas purifying rate is obtained, the generated gas such as NH3, H2, O2 or the like can serve as a raw material for other industrial production, high-toxicity high-risk byproducts are prevented being generated in the purifying process, and byproducts with higher added values can be obtained by an enriching and depositing method.
Owner:KUNMING UNIV OF SCI & TECH
Pre calcination additives for mixed metal oxide ammoxidation catalysts
ActiveUS20140148610A1High hydrogen cyanide productionImprove utilization efficiencyOrganic compound preparationHeterogenous catalyst chemical elementsGas phaseNitrogen
A process for preparation of catalysts for the production of acrylonitrile, acetonitrile and hydrogen cyanide comprising contacting at an elevated temperature, propylene, ammonia and oxygen in the vapor phase in the presence of a catalyst, said catalyst comprising a complex of metal oxides wherein a heat-decomposable nitrogen containing compound is added during the process for the preparation of the catalyst.
Owner:INEOS EURO LTD
Method for producing cyanogen-halide, cyanate ester compound and method for producing the same, and resin composition
ActiveUS20150299110A1Inhibit side effectsEfficient productionOrganic chemistryDecorative surface effectsCyanogen halideCyanide
A method for efficiently producing a cyanogen halide with suppressed side effects, and a method for producing a high-purity cyanate ester compound at a high yield includes contacting a halogen molecule with an aqueous solution containing hydrogen cyanide and / or a metal cyanide, so that the hydrogen cyanide and / or the metal cyanide is allowed to react with the halogen molecule in the reaction solution to obtain the cyanogen halide, wherein more than 1 mole of the hydrogen cyanide or the metal cyanide is used based on 1 mole of the halogen molecule, and when an amount of substance of an unreacted hydrogen cyanide or an unreacted metal cyanide is defined as mole (A) and an amount of substance of the generated cyanogen halide is defined as mole (B), the reaction is terminated in a state in which (A):(A)+(B) is between 0.00009:1 and 0.2:1.
Owner:MITSUBISHI GAS CHEM CO INC
Process for making nitriles
ActiveUS20130211121A1Reduce nickel contentOrganic compound preparationChemical recyclingCatalyst degradationHydrogen cyanide
Owner:INV NYLON CHEM AMERICAS LLC
Method for preparing 3-(methylthio)propanal
ActiveUS7119233B2High selectivityMinimizing high-boiling by-productsCarboxylic acid nitrile preparationOrganic compound preparationAcroleinCombinatorial chemistry
Owner:EVONIK DEGUSSA GMBH
High efficiency ammoxidation process and mixed metal oxide catalysts
ActiveCN102892496AHeterogenous catalyst chemical elementsCatalyst activation/preparationPolymer scienceAcetonitrile
A process and novel catalyst for the production of acrylonitrile, acetonitrile and hydrogen cyanide characterized by the relative yields of acrylonitrile, acetonitrile and hydrogen cyanide produced in the process and by the catalyst, which are defined by the following: a = [(%AN + (3 * %HCN) + (1.5 * %ACN)) %PC] * 100 wherein: %AN is the Acrylonitrile Yield and %AN = 81, %HCN is the Hydrogen Cyanide Yield, %ACN is the Acetonitrile Yield, %PC is the Propylene Conversion, and a is greater than 100.
Owner:INEOS EURO LTD
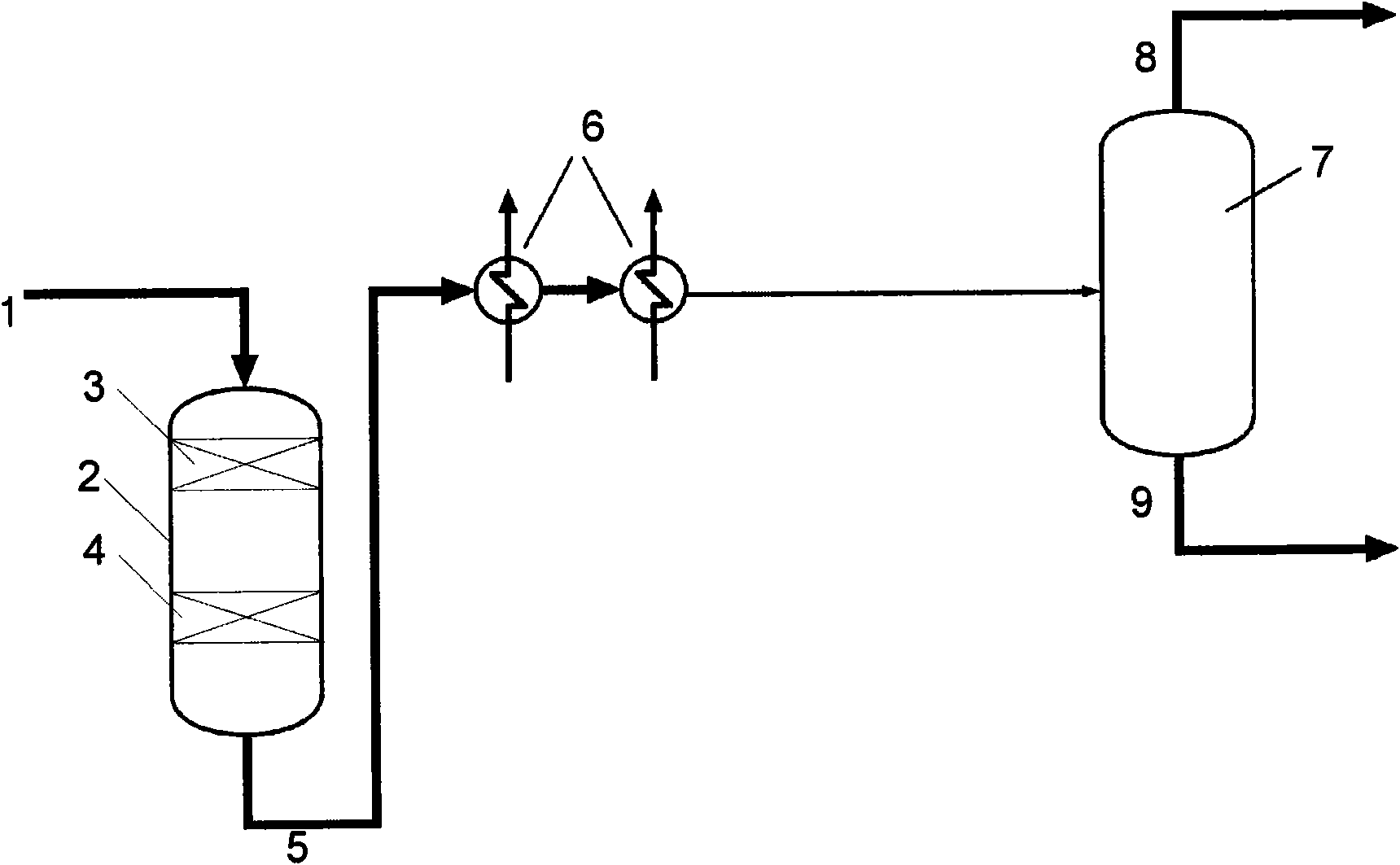
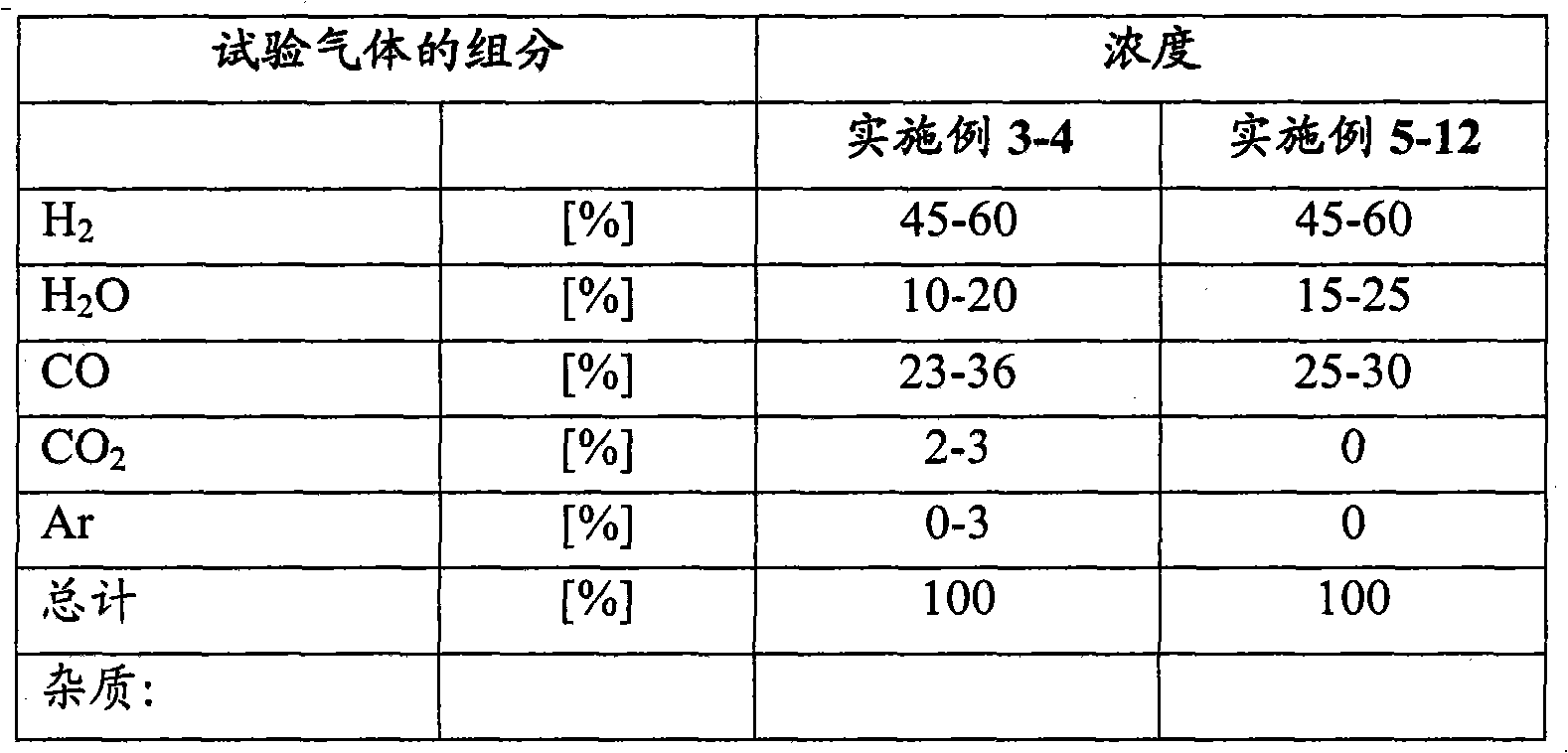
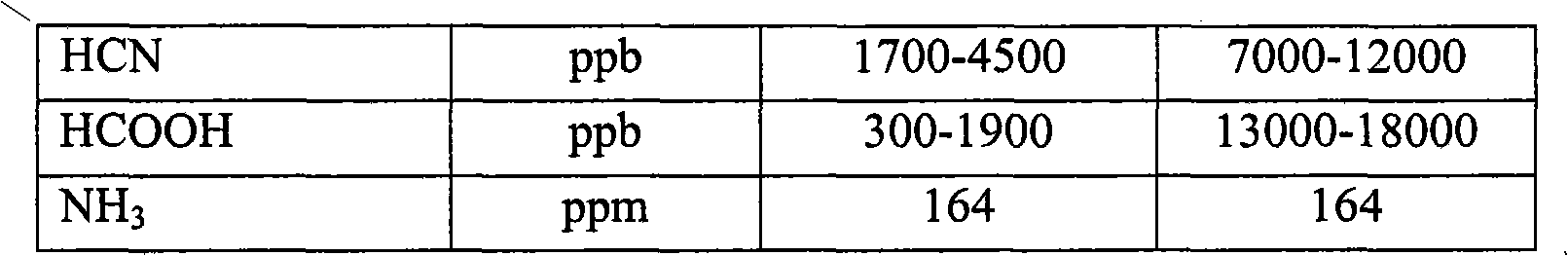
Process for a reduction in the amount of sulphur compounds, hydrogen cyanide and formic acid in synthesis gas
InactiveCN101918111AGas treatmentHydrogen separation using solid contactPtru catalystSulphur compound
A process for a reduction in the amount of sulphur compounds, hydrogen cyanide, formic acid and formic acid derivatives in synthesis gas comprising these compounds, the process comprising contacting the synthesis gas with a sulphur absorbent comprising material and thereafter with a catalyst comprising one or more metals selected from the group consisting of silver, gold, copper, palladium, platinum and their mixtures and supported on a carrier comprising at least one of the oxides of scandium, yttrium, lanthanum, cerium, titanium, zirconium, aluminium, zinc, chromium and molybdenum.
Owner:HALDOR TOPSOE AS
Process for making nitriles
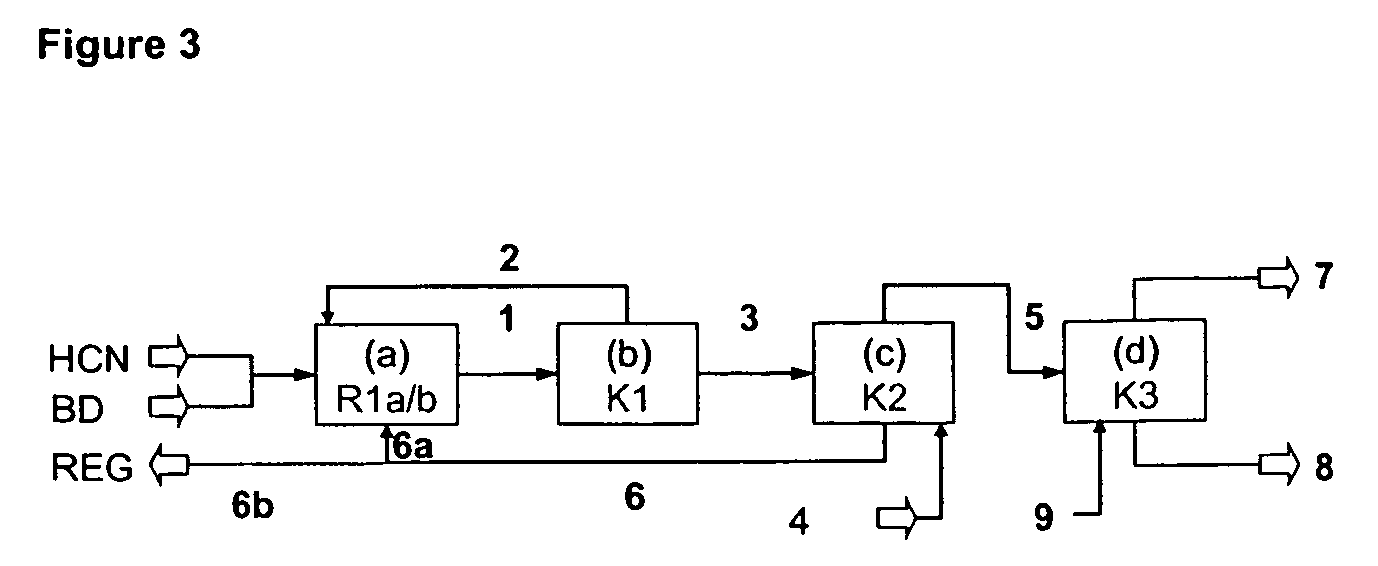
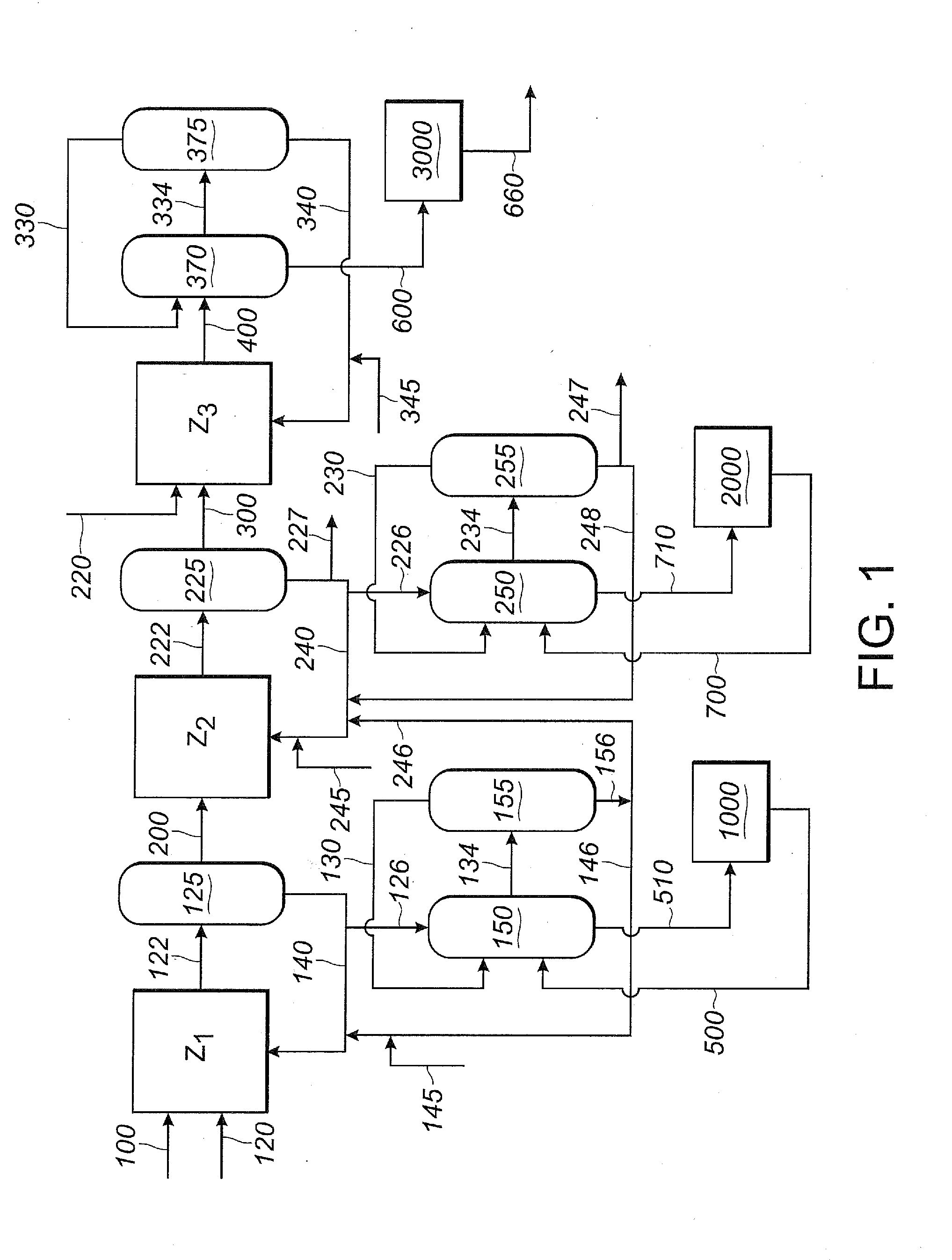
ActiveUS20130211122A1Reduce nickel contentOrganic compound preparationChemical recyclingPtru catalystIsomerization
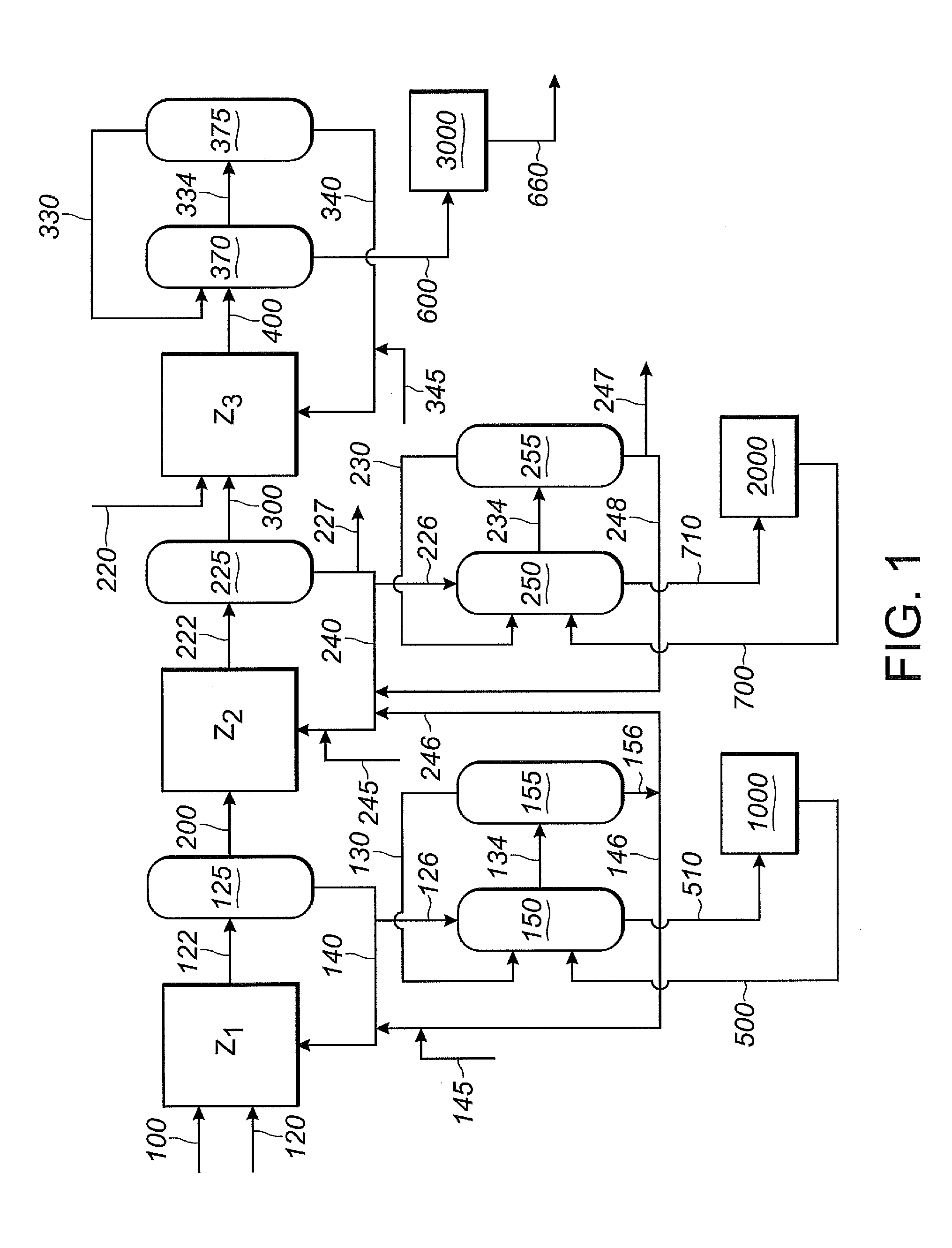
An improved multi-reaction zone process provides for improved nitrile product quality and yield. In a first reaction zone, 1,3-butadiene is reacted with hydrogen cyanide in the presence of a catalyst to produce pentenenitriles comprising 3-pentenenitrile and 2-methyl-3-butenenitrile. In a second reaction zone, 2-methyl-3-butenenitrile, recovered from the first reaction zone, is isomerized to 3-pentenenitrile. In an optional third reaction zone, 3-pentenenitrile recovered from the first and second reaction zones is reacted with hydrogen cyanide in the presence of a catalyst and a Lewis acid to produce adiponitrile. A portion of the first catalyst is purified and recycled. Zero valent nickel is added to the purified first catalyst before it is recycled.
Owner:INV NYLON CHEM AMERICAS LLC
Process for making nitriles
ActiveUS20130211125A1Minimize degradationMinimizes problemOrganic compound preparationChemical recyclingButadiene DioxideHCN poisoning
Adiponitrile is made by reacting 3-pentenenitrile with hydrogen cyanide. The 3-pentenenitrile is made by reacting 1,3-butadiene with hydrogen cyanide. The 1,3-butadiene feed includes a small amount of tertiary-butylcatechol. The catalyst for the reaction of 1,3-butadiene with hydrogen cyanide to make 3-pentenenitrile is recycled. At least a portion of the recycled catalyst is purified by an extraction process.
Owner:INV NYLON CHEM AMERICAS LLC
Process for making nitriles
ActiveUS20130211127A1Reduce nickel contentOrganic compound preparationChemical recyclingReaction zoneMethyl group
An improved multi-reaction zone process provides improved nitrile product quality and yield. In a first reaction zone, 1,3-butadiene is reacted with hydrogen cyanide in the presence of a catalyst to produce pentenenitriles comprising 3-pentenenitrile and 2-methyl-3-butenenitrile. In a second reaction zone, 2-methyl-3-butenenitrile, recovered from the first reaction zone, is isomerized to 3-pentenenitrile. In a third reaction zone, 3-pentenenitrile recovered from the first and second reaction zones is reacted with hydrogen cyanide in the presence of a catalyst and a Lewis acid to produce adiponitrile. Unwanted production and build-up of dinitriles, including methylglutaronitrile, in the first reaction zone for the hydrocyanation of 1,3-butadiene is prevented by limiting the flow of Lewis acid into the first reaction zone.
Owner:INV NYLON CHEM AMERICAS LLC
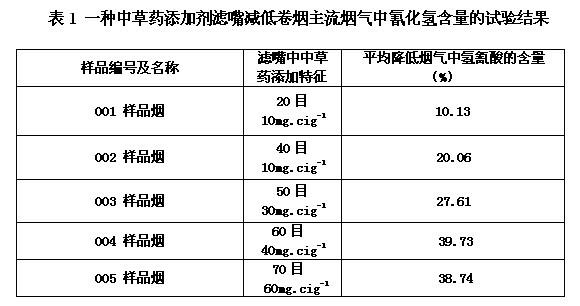
Chinese herbal additive for reducing hydrogen cyanide in main stream smoke of cigarette, preparation method and application thereof
ActiveCN102551207AReduce hydrogen cyanide contentStrong alkalineTobacco smoke filtersBiotechnologySmoked Plum
The invention relates to a Chinese herbal additive for reducing hydrogen cyanide in main stream smoke of cigarette, and a preparation method and application of the Chinese herbal additive. The Chinese herbal additive comprises Endoconcha Sepiae, limonite, and smoked plum, wherein the weight ratio of limonite to Endoconcha Sepiae is (1 to 10):100, and the weight ratio of smoked plum to Endoconcha Sepiae is (1 to 6):100. The preparation method comprises: (1) preparing the mixture powder of limonite and Endoconcha Sepiae; (2) preparing smoked plum extract; (3) preparing additive powder; and (4) preparing the Chinese herbal additive. The Chinese herbal additive provided by the invention can remove hydrogen cyanide in smoke, and Endoconcha Sepiae has strong alkalinity, high activity, large adsorption area and high adsorption effect, and has effects in neutralizing and occluding hydrogen cyanide.
Owner:CHINA TOBACCO JIANGXI IND CO LTD
Coal gas sampling method for determining content of hydrogen cyanide in coal gas
InactiveCN102607900AReduce sulfide contentSimple methodChemical analysis using titrationWithdrawing sample devicesPotassium hydroxideCadmium acetate
The invention provides a coal gas sampling method for determining the content of hydrogen cyanide in coal gas. The coal gas sampling method includes steps of leading coal gas to pass through cadmium acetate liquor so as to absorb hydrogen sulfide in the coal gas; then leading the coal gas to pass through potassium hydroxide liquor twice respectively to absorb the hydrogen cyanide in the coal gas so as to obtain sample absorption liquid absorbed with the hydrogen cyanide; leading the coal gas to pass through sulfuric acid liquor to absorb ammonia in the coal gas so as to protect a flow meter; and finally, leading the coal gas to pass through saturated picric acid liquor to absorb naphthalene in the coal gas and leading the coal gas to pass through the flow meter to obtain coal gas sampling quantity. After the sample absorption liquid absorbed with the hydrogen cyanide is inspected, the content of the hydrogen cyanide in the coal gas is obtained by means of conventional calculation. The coal gas sampling method effectively resolves the problem that as the content of sulfion in absorption liquid for determining the content of the hydrogen cyanide in the prior art is high, the content of the hydrogen cyanide in coal gas cannot be determined by a silver nitrate titration method. In addition, the coal gas sampling method is simple and practicable, and is easy in implementation.
Owner:云南昆钢煤焦化有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com