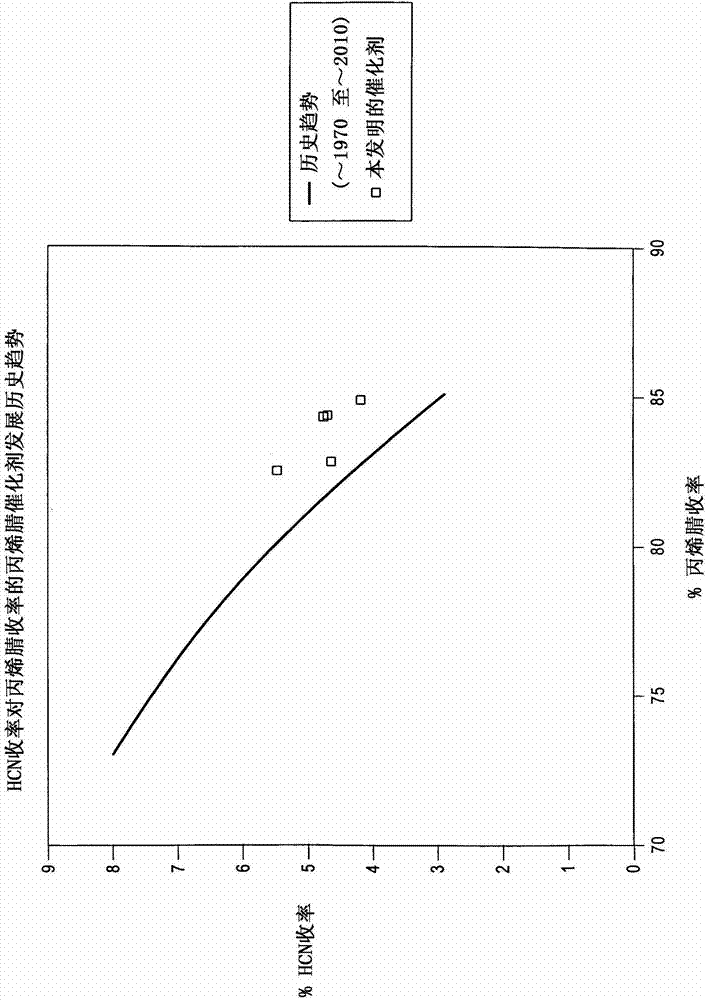
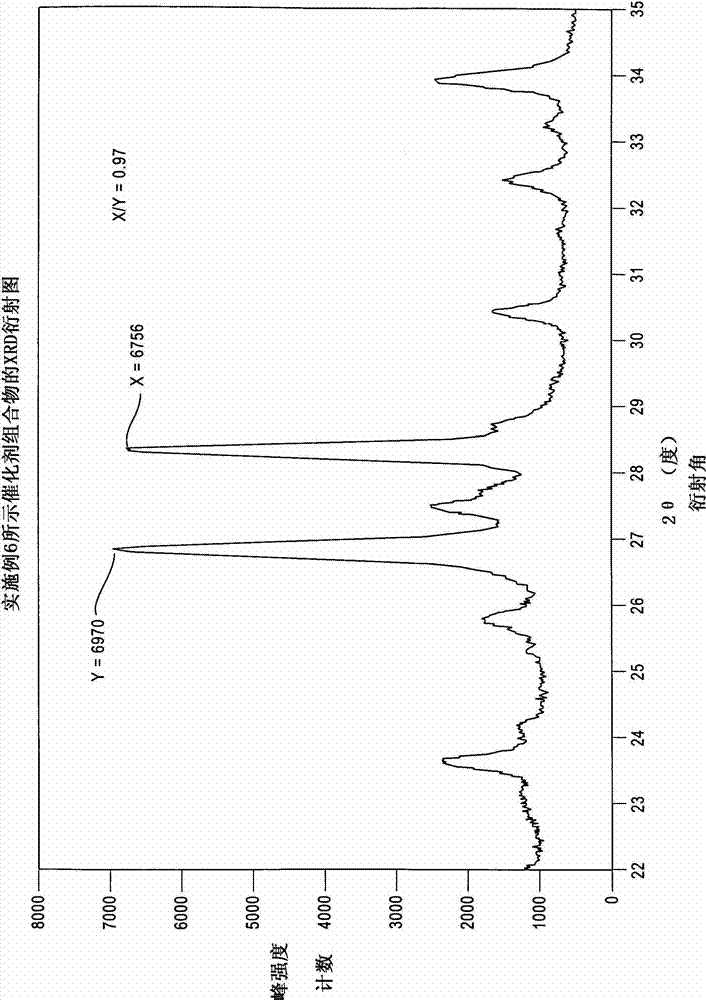
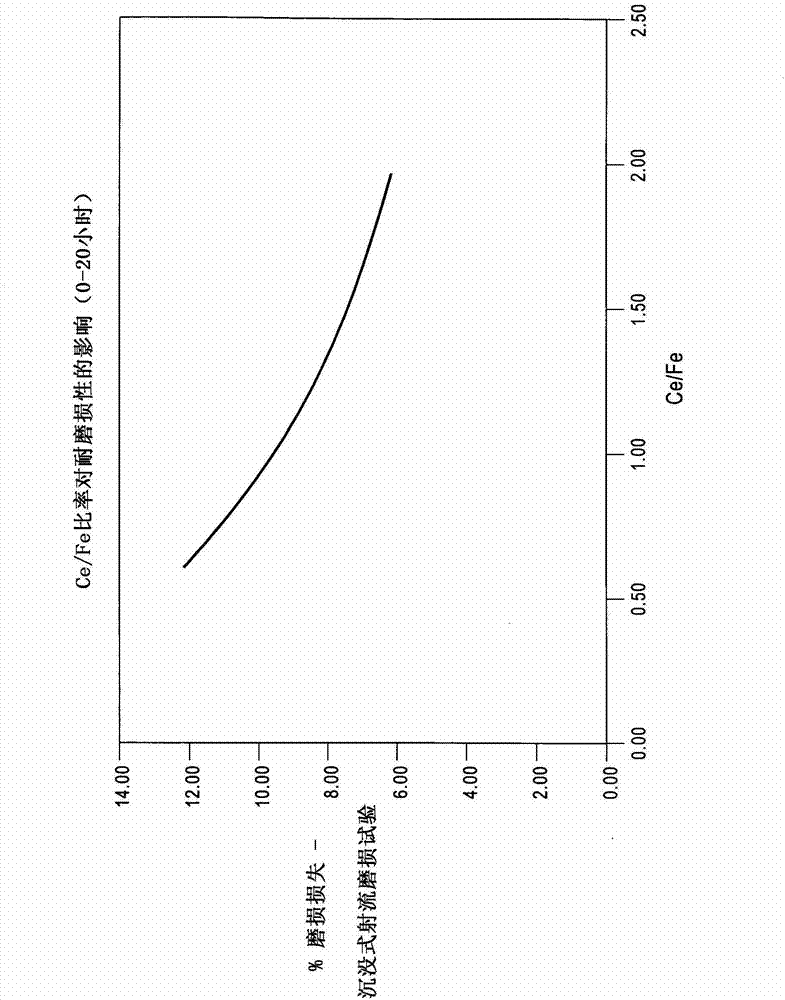
High efficiency ammoxidation process and mixed metal oxide catalysts
A catalyst and oxide technology, applied in metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, hydrocarbon ammoxidation preparation, etc., can solve complex problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0139] To illustrate the present invention, catalysts prepared according to the present invention were evaluated and compared under similar reaction conditions with similar catalysts prepared by prior art methods outside the scope of the present invention. These examples are provided for illustrative purposes only.
[0140] Composition Cs was prepared by various preparation methods as described below 0.1 K 0.1 Ni 5 Mg 2 Na 0.05 Fe 1.8 Bi 0.45 Ce 1.1 Mo 12.55 o 50.35 +45wt%NaSiO 2 and tested for the ammoxidation of propylene to acrylonitrile in a laboratory-scale reactor. All tests were performed in a 40cc fluidized bed reactor. Propylene was fed to the reactor at a rate of 0.06 WWH (ie weight propylene / weight catalyst / hour). The pressure in the reactor was maintained at 10 psig. The propylene / ammonia / air molar ratio is approximately 1 / 1.2 / 9.5. The reaction temperature is 430°C. After a stable period of ~20+ hours, a sample of the reaction product was collected. ...
Embodiment 1
[0155] Embodiment 1: Prepared according to the present invention
[0156] Reaction mixture A was prepared by heating 198 ml of deionized water to 65°C and then adding ammonium heptamolybdate (180.4 g) with stirring over 30 minutes to form a clear colorless solution. Silica sol (692 g, 32.5 wt% silica) was then added with stirring.
[0157] Heat 33ml of deionized water to 55°C, then add Fe(NO 3 ) 3 9H 2 O(73.6g), Ni(NO 3 ) 2 ·6H 2O(147.1g) and Mg(NO 3 ) 2 ·6H 2 O (51.9 g), to prepare reaction mixture B.
[0158] Reaction mixture C was prepared by heating 48ml of deionized water to 65°C and then adding ammonium heptamolybdate (43.75g) with stirring over 30 minutes to form a clear colorless solution.
[0159] By (i) 122.0g 50wt% (NH 4 ) 2 Ce(NO 3 ) 6 The aqueous solution was heated to 55°C, and (ii) while stirring and heating the solution, Bi(NO 3 ) 3 ·5H 2 O (22.1g), CsNO 3 (1.97g), KNO 3 (1.02g) and NaNO 3 (0.43 g), forming a clear orange solution to prepare...
Embodiment 2
[0164] Embodiment 2: Prepared according to the present invention
[0165] Reaction mixture A was prepared by heating 198 ml of deionized water to 65°C and then adding ammonium heptamolybdate (180.4 g) with stirring over 30 minutes to form a clear colorless solution.
[0166] Heat 33ml of deionized water to 55°C, then add Fe(NO 3 ) 3 9H 2 O(73.6g), Ni(NO 3 ) 2 ·6H 2 O(147.1g) and Mg(NO 3 ) 2 ·6H 2 O (51.9 g), to prepare reaction mixture B.
[0167] Reaction mixture C was prepared by heating 48ml of deionized water to 65°C and then adding ammonium heptamolybdate (43.75g) with stirring over 30 minutes to form a clear colorless solution.
[0168] By (i) 122.0g 50wt% (NH 4 ) 2 Ce(NO 3 ) 6 The aqueous solution was heated to 55°C, and (ii) while stirring and heating the solution, Bi(NO 3 ) 3 ·5H 2 O (22.1g), CsNO 3 (1.97g), KNO 3 (1.02g) and NaNO 3 (0..43 g), forming a clear orange solution, to prepare reaction mixture D.
[0169] Reaction mixture B was added to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com