Method for preparing adiponitrile
A technology of adiponitrile and pentenenitrile, which is applied in the field of preparation of adiponitrile, can solve problems that need to be improved, and achieve the effects of low cost, high purity, and simple and easy-to-operate process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
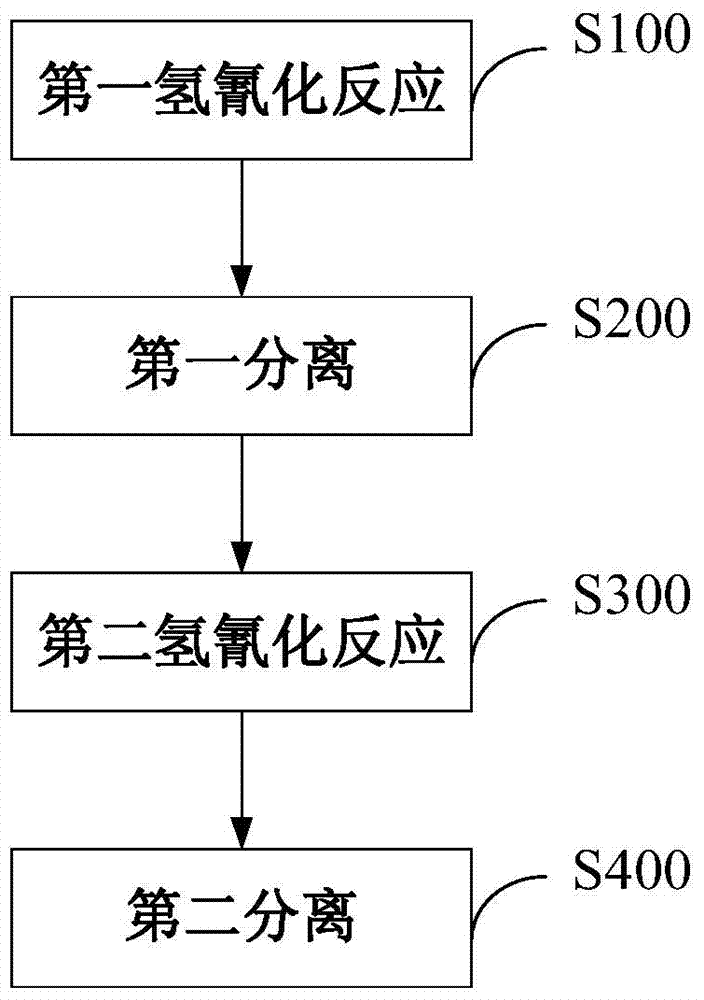
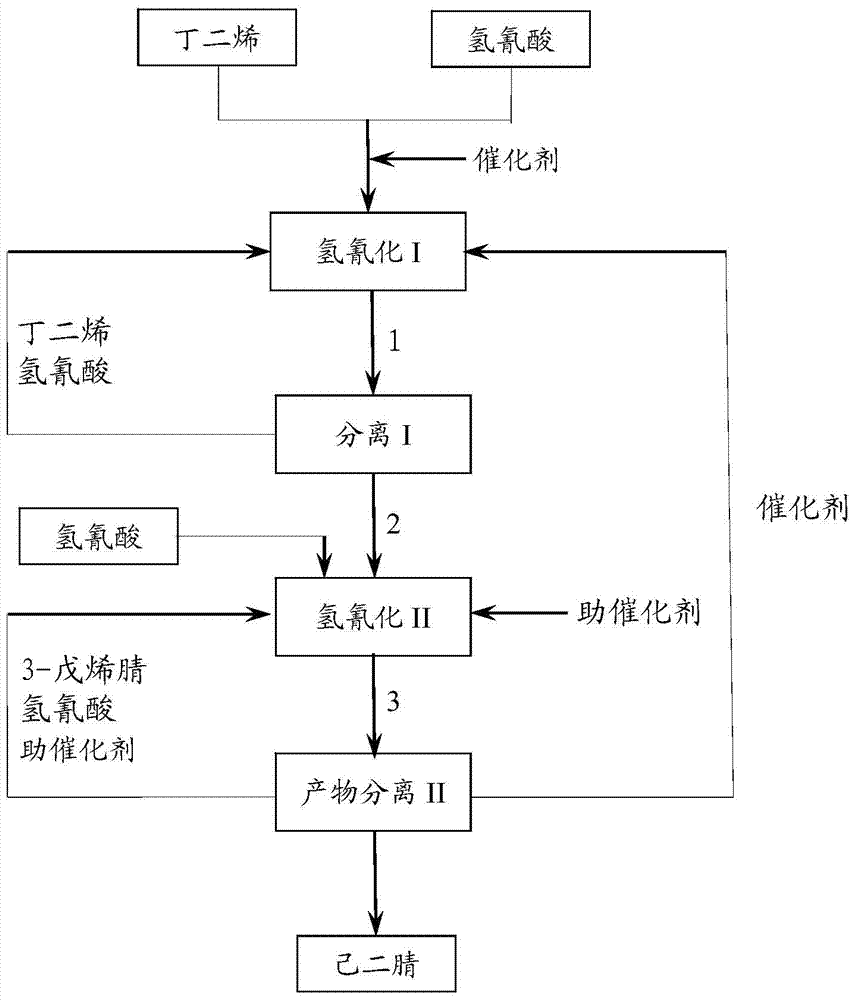
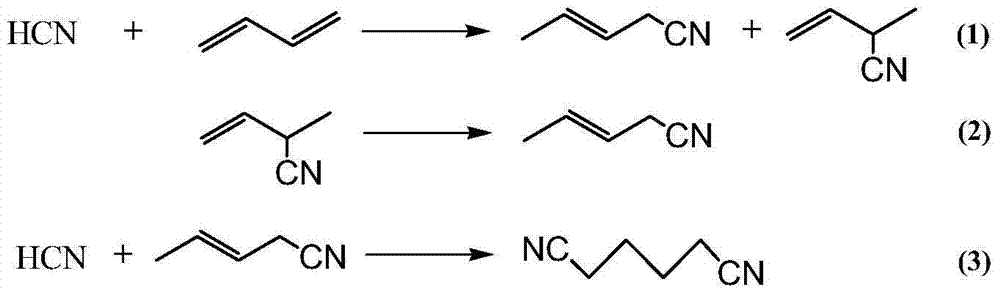
[0054] a) Hydrocyanide I: A complex composed of monodentate phosphine (tri-m-cresyl phosphite) and bidentate phosphine (compound represented by formula I) and zero-valent nickel (in monodentate phosphine and bidentate phosphine with zero In the complex composed of valent nickel, the molar ratio of monodentate phosphine: bidentate phosphine: zero-valent nickel is 6:2:1), and the molar ratio of HCN (hydrogen cyanide) and BD (butadiene) is 6:115. : 165 was put into the tank reactor, the controlled reaction temperature was 100° C., the pressure was 1.0 MPa, and the reaction residence time was 4 hours, so as to obtain the mixture 1 containing 3-pentenenitrile.
[0055]
[0056] b) Separation I: The resulting mixture 1 is introduced into separation I (rectification column). The rectification tower adopts a rectification tower with 50 trays, wherein the top pressure of the rectification tower is 0.05 MPa, the bottom pressure is 0.06 MPa, the temperature at the top is 40 degrees Ce...
Embodiment 2
[0062]a) Hydrocyanide I: A complex composed of monodentate phosphine (tri-o-cresyl phosphite) and bidentate phosphine (compound shown in formula II) and zero-valent nickel (in monodentate phosphine and bidentate phosphine with zero In the complex composed of valent nickel, the molar ratio of monodentate phosphine: bidentate phosphine: zero-valent nickel is 5:5:1), HCN and BD are put into the tank reactor with a molar ratio of 6:115:165, and the control The reaction temperature was 70° C., the pressure was 1.8 MPa, and the reaction residence time was 4 hours, in order to obtain a mixture 1 containing 3-pentenenitrile.
[0063]
[0064] b) Separate I:
[0065] Same as Example 1.
[0066] c) Hydrocyanation II: The above heavy component mixture 2 is introduced into the tank reactor of hydrocyanation II to carry out further 3-pentenenitrile hydrocyanation reaction.
[0067] Then, in the tank reactor, the co-catalyst triphenylboron is added according to the molar ratio of co-ca...
Embodiment 3
[0072] a) Hydrocyanide I: A complex composed of monodentate phosphine (tri-p-cresyl phosphite) and bidentate phosphine (compound represented by formula III) and zero-valent nickel (in monodentate phosphine and bidentate phosphine with zero In the complex composed of valent nickel, the molar ratio of monodentate phosphine: bidentate phosphine: zero-valent nickel is 7:2:1), HCN and BD are put into the tank reactor with a molar ratio of 6:115:165, and the control The reaction temperature was 90° C., the pressure was 1.8 MPa, and the reaction residence time was 4 hours, in order to obtain a mixture 1 containing 3-pentenenitrile.
[0073]
[0074] b) Separate I:
[0075] Same as Example 1.
[0076] c) Hydrocyanation II: The above heavy component mixture 2 is introduced into the tank reactor of hydrocyanation II to carry out further 3-pentenenitrile hydrocyanation reaction.
[0077] Then, into the tank reactor, the cocatalyst ZnCl 2 According to the molar ratio of hydrocyanic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com