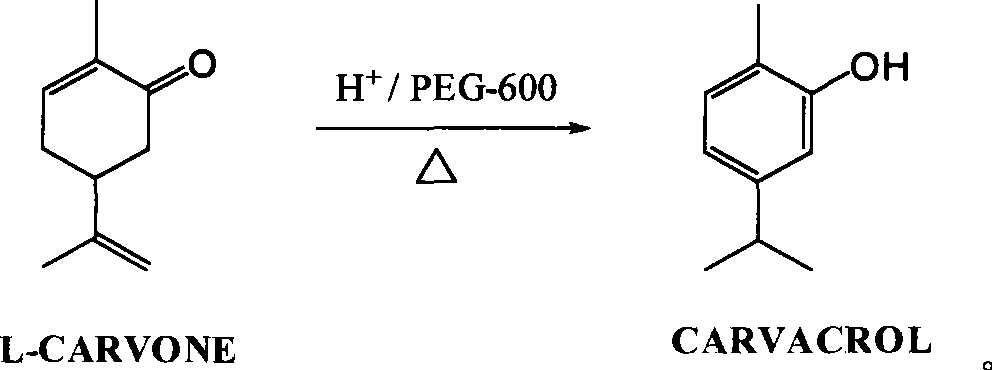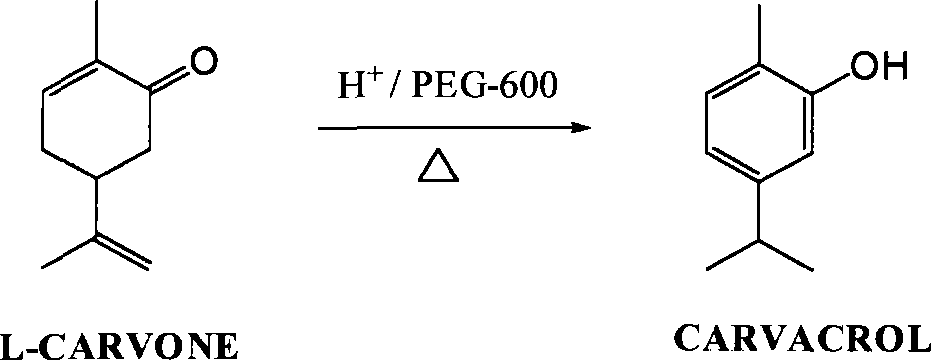Green synthesis of high-content carvacrol capable of replacing natural origanum
A technology of green synthesis and carvacrol, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of harmful working environment and high toxicity of dichloroethane, and achieve high product yield and convenient operation , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Put 130ml of phosphoric acid solution with a mass concentration of 45%, 100ml of L-carvone (about 96 grams) with a mass content of 99.25%, and 1.8 grams of PEG-600 into a 500ml three-necked flask, heat the oil bath to 102°C, keep the temperature for 9.5 hours, and take a sample GC analysis shows that 0.035% of L-carvone remains, the reaction is terminated by lowering the temperature, the stratification is allowed to stand, and the acid liquid phase in the lower layer is recycled; the organic phase in the upper layer is neutralized to PH=6.4 with a 5% sodium carbonate aqueous solution with a mass concentration of 5%, and the organic phase is reused Wash with saturated brine until neutral, dry over anhydrous magnesium sulfate, and distill under reduced pressure to obtain 93.4 g of carvacrol with a content of 99.85% and a yield of 98.1%.
Embodiment 2
[0019] Put 120ml of sulfuric acid solution with a mass content of 43%, 100ml of L-carvone (about 96 grams) with a mass content of 99.25%, and 1.9 grams of PEG-400 into a 500ml three-necked flask, heat the oil bath to 108°C, keep the temperature for 8 hours, and take a sample GC analysis shows that L-carvone remains at 0.027%, the reaction is terminated by cooling down, the layer is allowed to stand for stratification, and the acid liquid phase in the lower layer is recycled; the organic phase in the upper layer is neutralized to PH=6.8 with a 5% aqueous solution of sodium carbonate, and the organic phase is then saturated with Wash with salt water until neutral, dry over anhydrous magnesium sulfate, and distill under reduced pressure to obtain 93.8 g of carvacrol with a content of 99.88% and a yield of 98.5%.
Embodiment 3
[0021] Put 100ml of methanesulfonic acid solution with a mass content of 40%, 100ml of L-carvone (about 96 grams) with a mass content of 99.25%, and 1.6 grams of PEG-600 into a 500ml three-necked flask, heat the oil bath to 105°C, and heat for 8.5 Hours, sampling GC analysis, L-carvone residual 0.01%, cooling to terminate the reaction, standing for stratification, the lower layer of acid liquid phase circulation; the upper organic phase with a mass concentration of 5% sodium carbonate aqueous solution to neutralize to PH = 7.0, organic The phase was washed with saturated brine until neutral, dried over anhydrous magnesium sulfate, and then distilled under reduced pressure to obtain 94.1 g of carvacrol with a content of 99.86% and a yield of 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com