Method for synthesizing gamma, delta-nonsaturated ketones compound
A ketone compound, unsaturated technology, applied in the field of preparing γ, δ-unsaturated ketones, can solve the problems of reduced catalytic efficiency, increased catalytic cost, acid loss, etc., and achieves high conversion rate and selectivity, selectivity and conversion The effect of high rate and uniform distribution of acid strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of 6,10-dimethyl-5-undecen-2-one
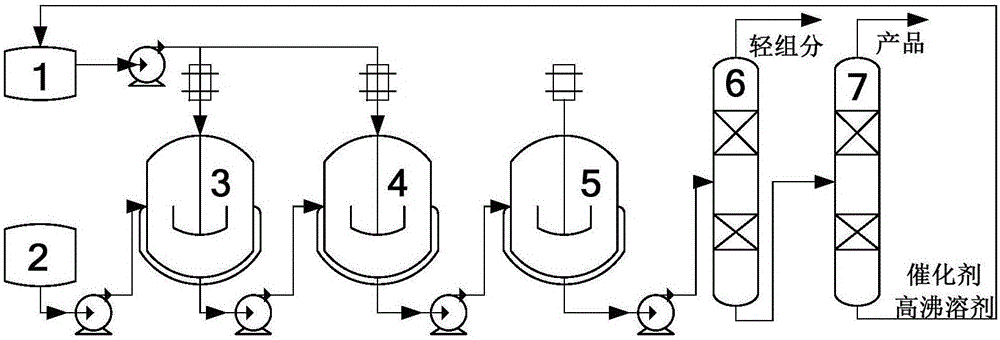
[0049] Add 1:3 equivalent mixed liquid of 3,7-dimethyl-1-octen-3-ol and 2-methoxypropene into the raw material storage tank (2), suck it into the first one at 0.33L / h In a closed 2L autoclave (3), the stirring speed was 400r / min, and the Bronsted acid ionic liquid catalyst ([STA][p-TsO], n=4) was dissolved in a high boiling solvent (26.5% biphenyl and 73.5% diphenyl phenylene ether azeotropic mixture) (mass fraction is 0.1kg / L), at 120 ° C, the catalyst solution is continuously injected into the first (3) and the second high-pressure Kettle (4). As shown in the flow chart, the obtained reaction materials are pumped into the next-stage reactor, and the mixture from the last-stage reactor enters the distillation tower (6) to remove light components. The light-removed product enters the simple rectification device (7) for separation to obtain the product and the high-boiling point solution of the catalyst respectiv...
Embodiment 2~9
[0053] According to the operation method of Example 1, different Bronsted acid ionic liquids are used to screen the synthetic 6-methyl-3,5-heptadien-2-one respectively, and the detection data obtained are shown in Table 1, wherein the conversion and product selectivities are calculated for 3,7-dimethyl-1-octen-3-ol.
[0054] The reaction conditions and the result of table 1 embodiment 1~9
[0055]
[0056]
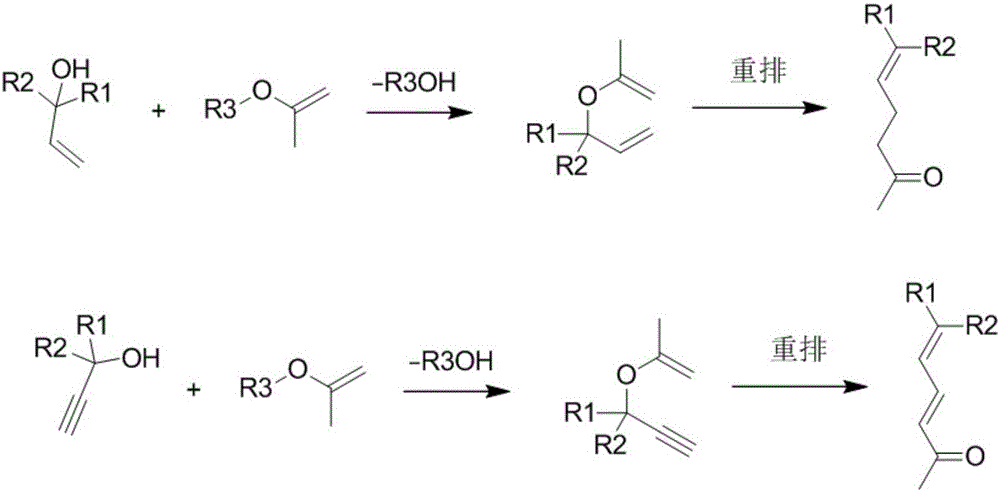
[0057] Alkynol rearrangement
Embodiment 10
[0058] Embodiment 10 prepares 6,10-dimethyl undeca-dien-2-one
[0059] Add 3,7-dimethyl-1-octyn-3-alcohol and 2-methoxypropene mixed liquid mixed at 1:3 equivalent in raw material storage tank (2), inhale into the first one at 0.33L / h In the airtight 2L autoclave (3), the stirring speed is 400r / min, the Bronsted acid catalyst ([SMI][p-TsO], n=4) is dissolved in the high boiling point solvent (mass fraction is 0.1kg / L), At 120°C, the catalyst solution was simultaneously and continuously injected into the first (3) and the second autoclave (4) through a pump at a rate of 0.3 ml / h. As shown in the flow chart, the obtained reaction materials are pumped into the next-stage reactor, and the mixture from the last-stage reactor enters the distillation tower (6) to remove light components. The light-removed product enters the simple rectification device (7) for separation to obtain the product and the high-boiling point solution of the catalyst respectively, and the obtained catalyst ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com