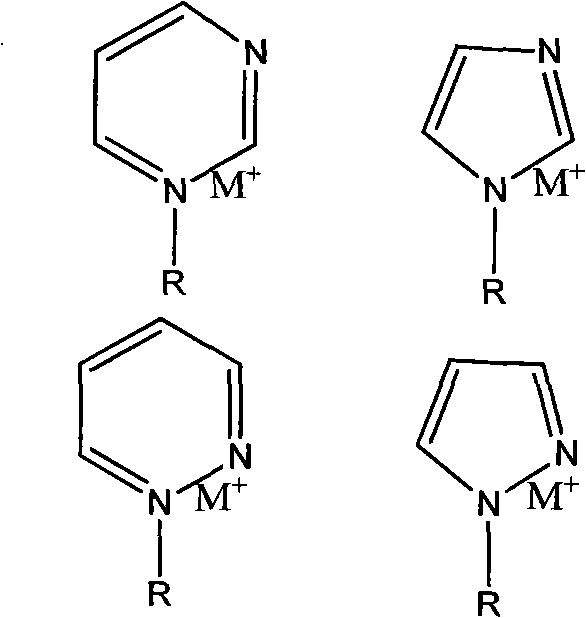
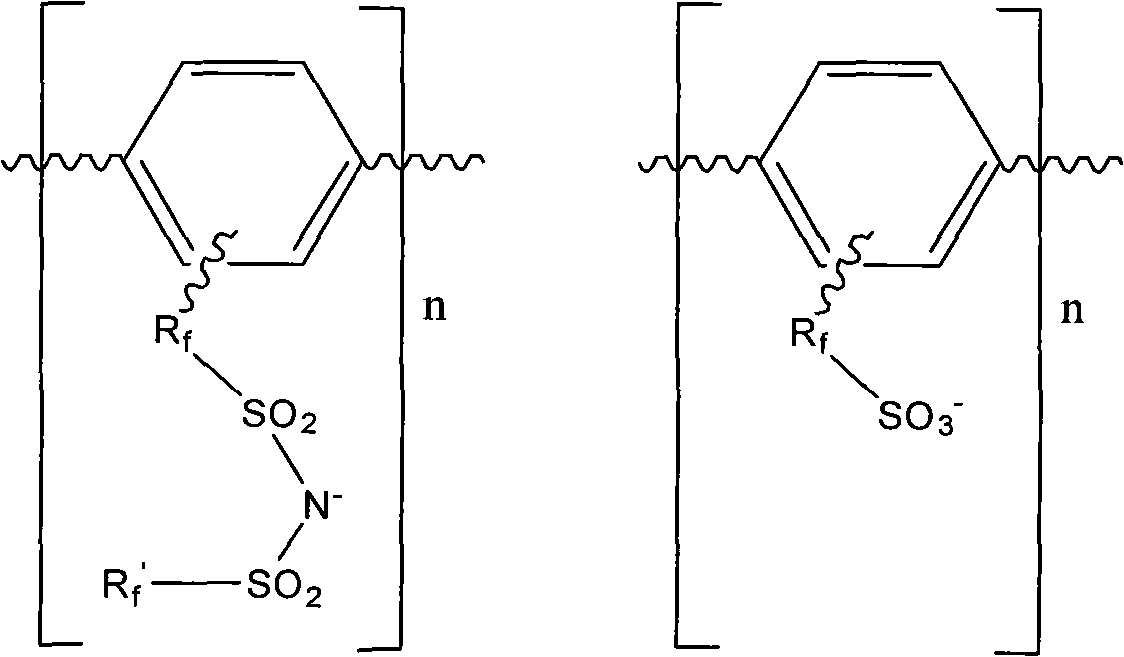
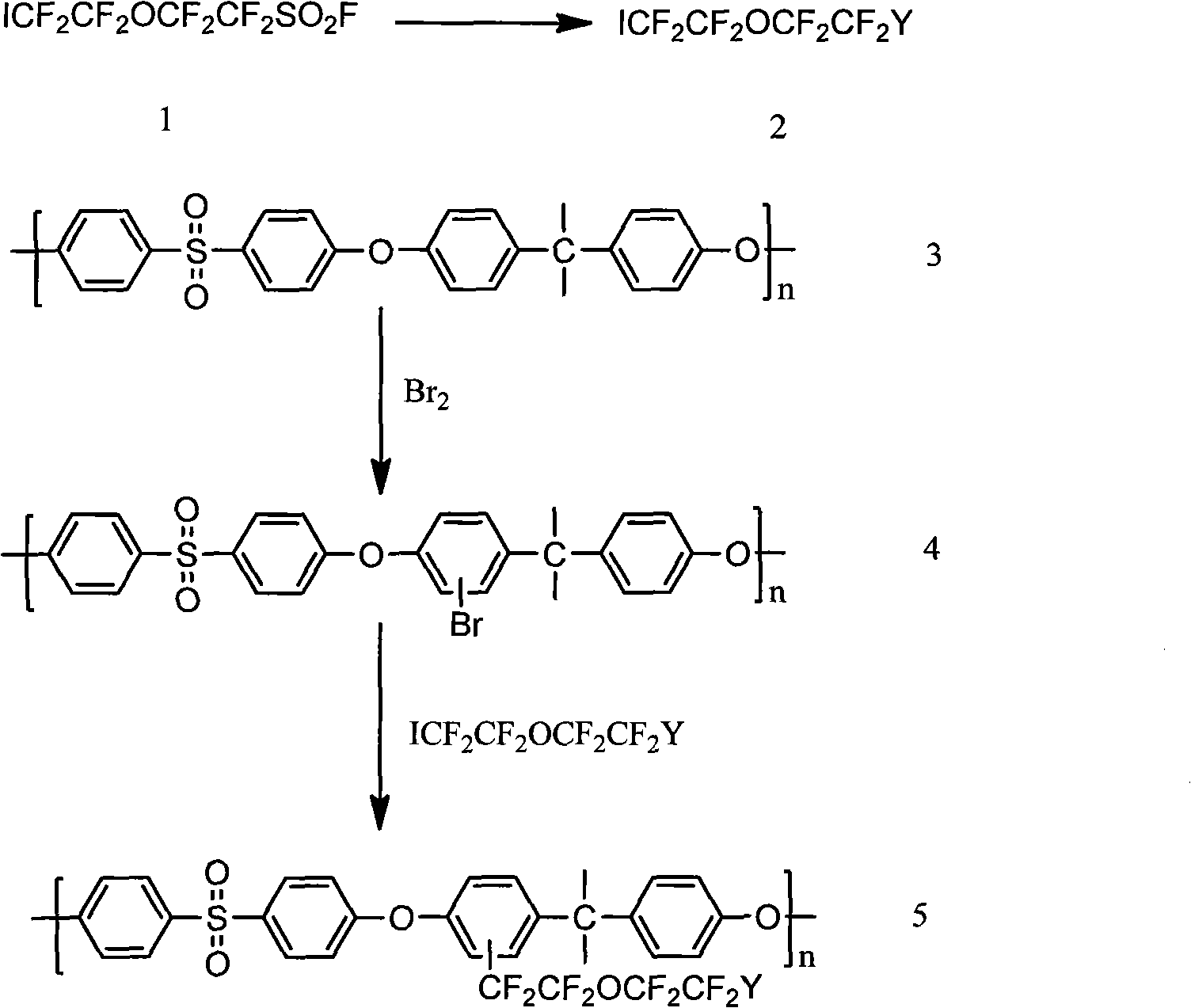
High-electric conductivity aromatic polymer ionic liquid diaphragm material and preparation method thereof
An aromatic polymer, ionic liquid technology, applied in organic diaphragms, chemical instruments and methods, circuits, etc., can solve the problems of methanol performance is not very high, ionic conductivity is low, unusable, etc., to achieve good ionic conductivity, strong Cationic activity, not easy to run off effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] A preparation method of a highly conductive aromatic polymer ionic liquid diaphragm material of the present invention mainly includes the following steps: Step 1: Synthesizing an active intermediate containing a fluorine-containing sulfonic acid group or a fluorine-containing sulfonimide
[0043] Synthesis of active intermediates containing fluorine-containing sulfonic acid groups, whose molecular formula is XR f SO 3 M, where X is iodine or bromine, M is a metal element, R f Refer to -C n f 2n -or-[CF 2 CF 2 ] n OCF 2 CF 2 -, n is an integer from 1 to 40, including 1 and 40; or an active intermediate for synthesizing fluorine-containing sulfonimide, whose molecular formula is XR f SO 2 NMSO 2 R f ’, where X is iodine or bromine, M is a metal element, R f Refer to -C n f 2n -or-[CF 2 CF 2 ] n OCF 2 CF 2 -, R f ’ refers to ZC n f 2n -, Z is hydrogen, fluorine or chlorine, n is an integer from 1 to 40, including 1 and 40;
[0044] Step 2: Synthesis ...
Embodiment 1
[0062] Raw materials: iodine-terminated fluoroalkyl ether group sulfonyl fluoride (ICF 2 CF 2 OCF 2 CF 2 SO 2 F) with polysulfone
[0063] Step 1: Synthesis of ICF 2 CF 2 OCF 2 CF 2 SO 3 K
[0064] In a 300ml beaker, 100mMol ICF 2 CF 2 OCF 2 CF 2 SO 2 F was slowly added to a solution containing 220mMolKOH, 30ml water and 10ml ethanol, stirred for 0.5 hours, then added 30ml ethyl acetate and 10ml water to completely dissolve the resulting yellow solid, separated the organic layer, and continued to use 20ml ethyl acetate after the aqueous layer After extraction, all organic solutions were combined, then evaporated to remove the solvent, and dried under vacuum at 70 °C for 8 hours to obtain ICF as a yellowish solid 2 CF 2 OCF 2 CF 2 SO 3 K.
[0065] Step 2: Synthesis of PSU-CF 2 CF 2 OCF 2 CF 2 SO 3 h
[0066] At room temperature, drop an appropriate amount of liquid bromine into a dichloromethane solution containing polysulfone and stir to generate brom...
Embodiment 2
[0074] Raw material is identical with embodiment 1, is the fluoroalkyl ether group sulfonyl fluoride (ICF of iodine terminal group) 2 CF 2 OCF 2 CF 2 SO 2 F) with polysulfone.
[0075] Step 1: Synthesis of ICF 2 CF 2 OCF 2 CF 2 SO 2 NMSO 2 CF 3
[0076] Add 12.2mMol dry KF and 2.57mMolCF to the 25ml flask 3 SO 2 NHNa, evacuate to 2 mHg at 100 °C, then add 1.27 g of ICF 2 CF 2 OCF 2 CF 2 SO 3 F and 5ml of dry acetonitrile, remove volatile substances in the mixture at -196°C, keep the temperature at 60-80°C, stir and react for two days, then distill off the solvent under reduced pressure, and dry in vacuo to obtain 2.36 grams of solid product ICF 2 CF 2 OCF 2 CF 2 SO 2 N(K)SO 2 CF 3 .
[0077] Step 2: Synthesis of PSU-CF 2 CF 2 OCF 2 CF 2 SO 2 N(K)SO 2 CF 3
[0078] At room temperature, drop an appropriate amount of liquid bromine into the dichloromethane solution containing polysulfone and stir to generate brominated polysulfone P(SU-mBr); add 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com