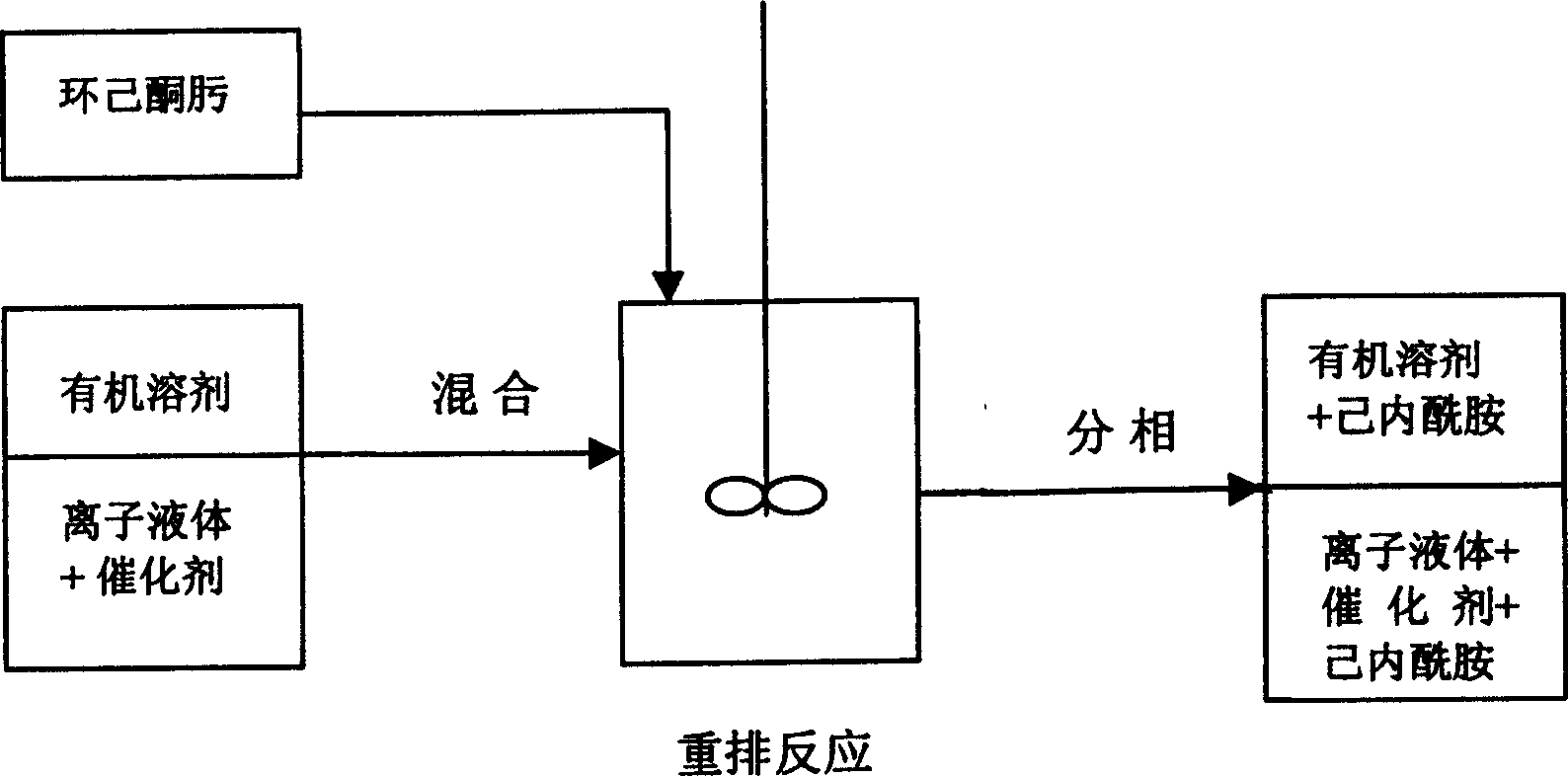
Process for preparation of caprolactam
A technology of cyclohexanone oxime and ionic liquid, which is applied in the field of preparation of caprolactam, can solve the problems of reaction rate control, difficulty in obtaining heat from the system, separation of difficult reaction products from the catalytic system, expensive ionic liquid, etc., and achieve high selectivity liquid phase Effects of Beckmann rearrangement reaction, energy consumption reduction and production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This example illustrates that the method provided by the present invention can be implemented in a hydrophilic ionic liquid under different catalyst to cyclohexanone oxime dosage ratios.
[0036] In a 100ml round bottom flask, add 2.0ml of hydrophilic ionic liquid [bmim][BF 4 ], 5.00ml toluene (product of Beijing Chemical Reagent Company, analytically pure) and 0.50ml POCl 3 (product of Beijing Chemical Reagent Company, analytically pure), the temperature of the oil bath is controlled at 80 ° C, and under magnetic stirring, cyclohexanone oxime-toluene solutions with different volumes and a concentration of 2.00 mol / L are added dropwise (cyclohexanone oxime is produced by Yueyang Yingshan Provided by the caprolactam factory, content > 99.5%), after 30 minutes of reaction, static phase separation, thereby completing the Beckmann rearrangement reaction.
[0037] Add 5.00ml of 10% ammonia water to the ionic liquid phase to quench the reaction, then extract twice with 5.00m...
Embodiment 2
[0039] This example illustrates that the method provided by the present invention can be implemented in hydrophilic ionic liquids with different catalyst dosages.
[0040] In a 100ml round bottom flask, add 2.0ml of hydrophilic ionic liquid [bmim][BF 4 ], 5.00ml toluene and different volumes of POCl 3 , the temperature of the oil bath is controlled at 80°C, and 10.00 ml of cyclohexanone oxime-toluene solution with a concentration of 1.00 mol / L is added dropwise under magnetic stirring, and the phase is separated after 30 minutes of static reaction, thereby completing the Beckmann rearrangement reaction.
[0041] The results analyzed according to the method of Example 1 are listed in Table 2.
[0042] Table 1.
[0043] Cyclohexanone oxime conversion with cyclohexanone oxime Selectivity, % Catalytic conversion number
[0044] Amount, ml Yield, % Cyclohexanone Caprolactam Others (TON)
[0045]5.00 99.77 0.00 98.59 1.41 1.83
[0046] 7.50 94.06 5.79 91.73 2.48 2.58
[0047] ...
Embodiment 3
[0057] This example illustrates that the method provided by the present invention can be implemented in hydrophilic ionic liquids at different dosages of ionic liquids.
[0058] In a 100ml round bottom flask, add different volumes of hydrophilic ionic liquid [bmim][BF 4 ], 5.00ml toluene and 0.50ml POCl 3 , the temperature of the oil bath is controlled at 80°C, under magnetic stirring, different volumes of cyclohexanone oxime-toluene solutions with a concentration of 2.00 mol / L are added dropwise, and after 30 minutes of reaction, the phases are separated at rest, thereby completing the Beckmann rearrangement reaction.
[0059] The results analyzed according to the method of Example 1 are listed in Table 3.
[0060] table 3.
[0061] Ionic Liquid Cyclohexanone Oxime Oxime Conversion Selectivity, % Catalytic Conversion Number
[0062] ml ml % Cyclohexanone Caprolactam Other (TON)
[0063] 2.0 7.50 91.52 5.43 91.58 2.99 2.51
[0064] 3.0 7.50 58.51 6.13 85.27 8.60 1.61
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com