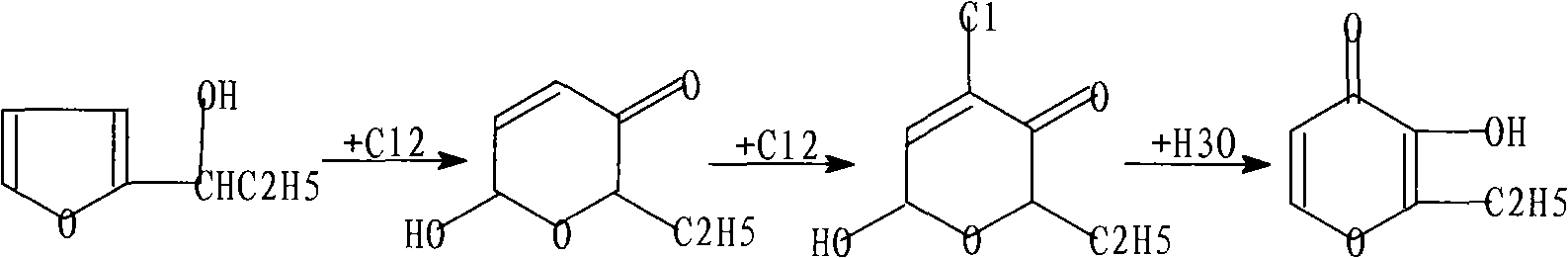
Ethyl maltol synthetic method
A technology of ethyl maltol and a synthesis method, applied in the field of chemistry, can solve problems such as environmental pollution, serious environmental pollution, consumption of sodium hydroxide, etc., and achieve the effects of reducing production costs and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Preparation of chlorination rearrangement reaction mixture:
[0033] Add 600mL of methanol aqueous solution (the mass fraction of methanol is 60%) into a 1000mL four-neck flask equipped with a stirrer, thermometer, dropping funnel and ventilation tube, cool to -25°C, and add α- A methanol solution of furan propanol (96mL of α-furan propanol with a density of 1.05g / mL and a content of 95.3% mixed with 104mL of methanol), at the same time, chlorine gas was introduced into the bottle at a certain ratio, and the reaction temperature was always maintained at -10~ -12°C. After the methanol solution of α-furan propanol is added dropwise, continue to pass chlorine gas for about 15 minutes until the reaction temperature drops significantly, stop the chlorine gas flow, and continue the reaction for 15 minutes to obtain a hydrolysis mixture containing the product ethyl maltol, with a volume of about 800 mL.
[0034] (2) Hydrolysis of chlorination rearrangement reaction mixtur...
Embodiment 2
[0044] (1) Preparation of chlorination rearrangement reaction mixture: same as Example 1.
[0045] (2) Hydrolysis of chlorination rearrangement reaction mixture:
[0046] Take 100mL of the chlorination rearrangement reaction mixture each time, and add them to five 125mL stainless steel (with polytetrafluoroethylene lining) hydrolysis reaction kettles respectively, and put the reaction kettles into 95°C and 105°C respectively. , 115°C, 125°C, 135°C, 140°C electric blast drying oven, under the hydrolysis pressure of 1.9, 2.6, 3.5, 4.7, 6.1, 6.8MPa, the hydrolysis reaction time is 3.0h, take out the reaction kettle Spray with tap water and be cooled to room temperature (need about 1h), separate the by-product methyl chloride gas that generates in the hydrolysis reaction, obtain the hydrolysis mixed solution that contains product ethyl maltol and measure the volume of hydrolyzate in each reactor ( The volume is 81-92mL).
[0047] Comparative example: with embodiment 1.
[0048]...
Embodiment 3
[0051] (1) Preparation of chlorination rearrangement reaction mixture:
[0052] Add 600 mL of aqueous methanol solutions with mass fractions of 50%, 60%, 70%, 80%, and 90% of methanol in a 1000 mL four-necked bottle equipped with a stirrer, thermometer, dropping funnel, and vent tube, and cool to - At 25°C, add a methanol solution of α-furan propanol drop by drop from the dropping funnel (96 mL of α-furan propanol with a density of 1.05 g / mL and a content of 95.3% is mixed with 104 mL of methanol), and at the same time, start to add a certain proportion to the bottle Chlorine gas is passed through the medium, and the reaction temperature is always maintained at -10 to -12°C. After the methanol solution of α-furan propanol was added dropwise, the chlorine gas flow was continued for about 15 minutes until the reaction temperature dropped significantly, the chlorine gas flow was stopped, and the reaction was continued for 15 minutes to obtain a hydrolysis mixture containing the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com