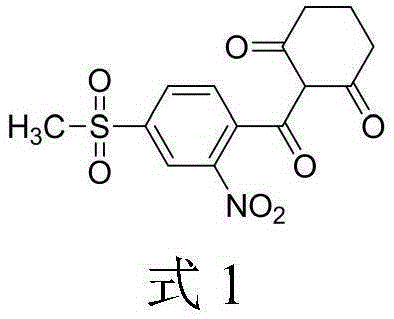
Synthesis method of mesotrione
A technology of mesotrione and its synthetic method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve complete reaction, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
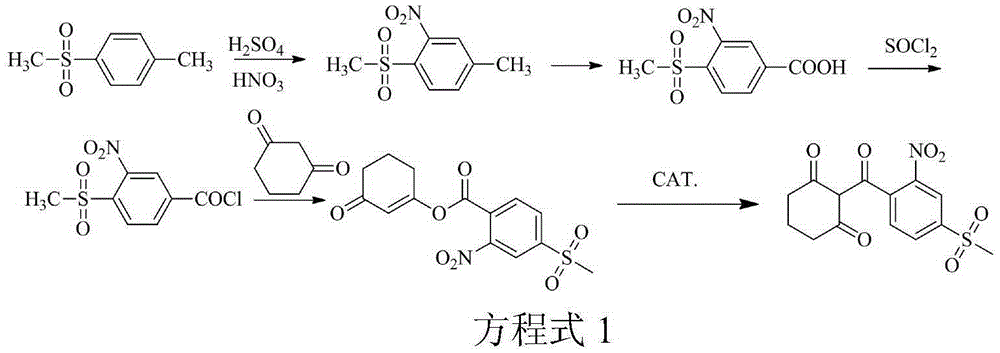
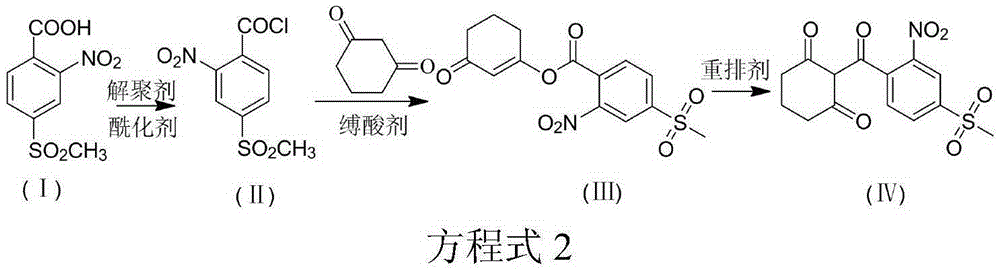
[0051] (1) Synthesis of acylate: Turn on the tail gas absorption system, add 185g (0.3eqv) of solid phosgene (acylating agent) and 450g of toluene to the four-neck flask, stir in a water bath and heat up to 70-80°C until the solid phosgene is complete Dissolve and keep warm for later use. Add 2-nitro-4-thiamphenicol benzoic acid 460g, toluene 1150g and trace 0.1g depolymerizing agent DMF (0.02wt%) in the acylation reaction flask meter), heated to 50°C (acylation reaction temperature) under stirring, and started to drop the pre-configured solid phosgene toluene solution. After the dropwise addition, continue the reaction for about 2 hours. , to obtain the acylation reaction solution.
[0052] (2) Synthesis of esterified product: Add 210g (1.0eqv) of cyclohexanedione and 154.3g (0.6eqv) of potassium carbonate (acid-binding agent) to the acylation reaction solution, heat up to 55°C, react for 8 hours, and cool down to obtain Esterification reaction solution.
[0053] (3) Synth...
Embodiment 2
[0056] Compared with Example 1, the difference is that thionyl chloride is used as an acylating agent, and the dosage equivalent of thionyl chloride is 1.2eqv. After the reaction treatment was completed, the chromatographic purity of the obtained mesotrione was 91.0%, and the yield was 86.6%.
Embodiment 3
[0058] (1) Synthesis of acylate: open the tail gas absorption system, add 446g (0.8eqv) of solid phosgene (acylating agent) and 450g of toluene to the four-neck flask, stir and raise the temperature to 70-80°C in a water bath until the solid phosgene is complete Dissolve and keep warm for later use. Add 2-nitro-4-thiamphenicol benzoic acid 460g, toluene 1150g and trace 0.2g depolymerizing agent DMF (0.04wt%) in the acylation reaction flask meter), heated to 70°C (acylation reaction temperature) under stirring, and started to drop the pre-configured solid phosgene toluene solution. After the dropwise addition, continue to react for about 2 hours. , to obtain the acylation reaction solution.
[0059] (2) Synthesis of esterified product: Add 273g (1.3eqv) of cyclohexanedione and 154.3g (0.6eqv) of potassium carbonate (acid-binding agent) to the acylation reaction solution, heat up to 55°C, react for 8 hours, and cool down to obtain Esterification reaction solution.
[0060] (3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com