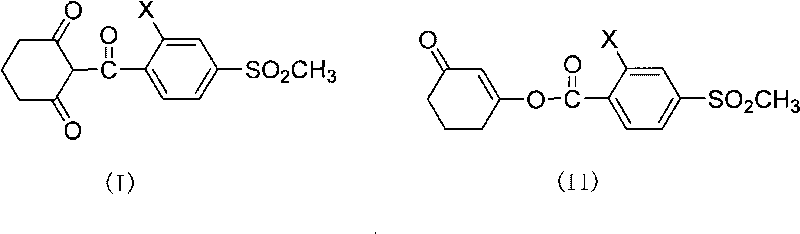
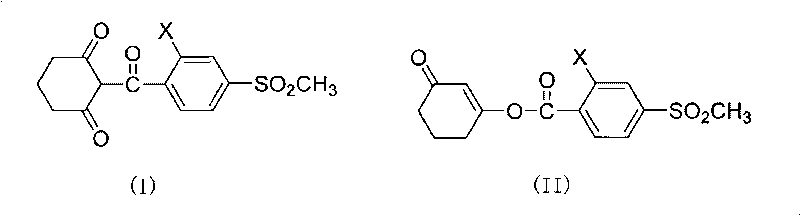
Composite method of triketone compound
A synthesis method and compound technology, applied in the synthesis of mesotrione and sulcotrione, can solve the problems of long reaction time and incomplete rearrangement reaction, and achieve the effects of high product yield, environmental friendliness and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of sulcotrione: In a three-necked flask, add 328g (1.0mol) 3-(2-chloro-4-thiamphenicol benzoyloxy)-2-cyclohexene-1-one, 2L 1,2 -Dichloroethane, 222.6g (2.2mol) triethylamine, 6.8g (0.05mol) 6-hydroxypurine, stirred, heated, the reaction temperature was 45-50°C, reacted for 5h, cooled, acidified with 10% hydrochloric acid, Make the pH value <1, statically separate the layers, and desolventize the organic phase to obtain 278 g of sulcotrione, with a yield of 85%.
Embodiment 2
[0021] Preparation of mesotrione: In a three-necked flask, add 339g (1.0mol) 3-(2-nitro-4-thiamphenicol benzoyloxy)-2-cyclohexen-1-one, 2.5L 1 , 2-dichloroethane, 222.6g (2.2mol) triethylamine, 6.8g (0.05mol) 6-hydroxypurine, stirring, heating, reaction temperature at 45-50°C, reaction for 5h, cooling, and 10% hydrochloric acid Acidify to make the pH value < 1, static layering, and desolvation of the organic phase to obtain 284.6 g of mesotrione, with a yield of 84%.
Embodiment 3-5
[0023] With reference to the method of Example 1, the consumption of triethylamine was changed, and other reaction conditions were the same as in Example 1 to obtain sulcotrione, and the results are shown in Table 1.
[0024] Table 1: Influence of the amount of triethylamine on the reaction
[0025] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com