Method for preparing progesterone and derivatives thereof
A technology of derivatives and progesterone, which is applied in the field of chemical preparation, can solve the problems of restricting large-scale industrial production, high raw material costs, and long synthetic routes, and achieve the effects of short synthetic routes, high product quality, and easy monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
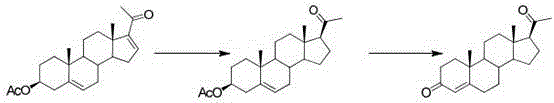
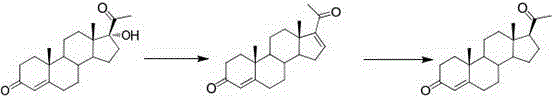
[0039] The first step, oxidation reaction: Add PCC (20g, 1W) and dichloromethane (200ml, 10V) to the dry three-necked flask, stir to dissolve, add silica gel (30g, 1.5W), under nitrogen protection, the internal temperature is controlled at 20~25 ℃, weigh compound 1 (20g, 1W) and dissolve it in dichloromethane (100ml, 5V), transfer it to the dropping funnel, and drop it into the three-necked bottle. The temperature during the dropping process should not exceed 25°C. , kept at this temperature for 5 hours. After the reaction was completed, filter, the filter cake was washed with dichloromethane (200ml, 10V), and the filtrate was concentrated under reduced pressure until dripping without solvent to obtain oxide 2 (18.8g). The weight yield was 94%. HPLC (High Performance Liquid Chromatography, high performance liquid phase chromatography) content of 98%.
[0040] The second step, rearrangement reaction: Add oxide 2 (18.8g, 1W) and DMF (56ml, 3V) into the reaction flask, stir and ...
Embodiment 2
[0042] The first step, oxidation reaction: add PDC (30g, 1.5W) and chloroform (200ml, 15V) to the dry three-necked flask, stir to dissolve, add silica gel (30g, 1.5W), under the protection of nitrogen, the internal temperature is controlled at 0~ 10°C, weigh compound 1 (20g, 1W) and dissolve it in chloroform (100ml, 5V), transfer it to the dropping funnel, and drop it into the three-necked bottle. Afterwards, the reaction was incubated at this temperature for 6 hours. After the reaction was completed, it was filtered, and the filter cake was washed with chloroform (200ml, 10V). The filtrate was concentrated under reduced pressure until dripping without solvent to obtain oxide 2 (18.0g). The weight yield was 90%, and the HPLC content was 96%.
[0043] The second step, rearrangement reaction: Add oxide 2 (18g, 1W) and dimethylacetamide (36ml, 2V) into the reaction flask, stir and control the temperature to 20°C, add 2,2'-bipyridine (0.02g , 0.001W), copper acetate (0.02g, 0.001...
Embodiment 3
[0045]The first step, oxidation reaction: Add PCC (40g, 2W) and 1,2-dichloroethane (400ml, 20V) to a dry three-necked flask, stir to dissolve, add silica gel (30g, 1.5W), under nitrogen protection, Control the temperature at 25~30°C, weigh compound 1 (20g, 1W) and dissolve it in 1,2-dichloroethane (200ml, 10V), transfer it to the dropping funnel, and drop it into the three-necked bottle. Not more than 30°C, after the dropwise addition is completed, keep the reaction at this temperature for 1 hour. After the reaction was completed, filter, the filter cake was washed with 1,2-dichloroethane (200ml, 10V), and the filtrate was concentrated under reduced pressure until no solvent was dropped, and the weight yield of oxide 2 (18.6g) was 93%, and the HPLC content was 92%. .
[0046] The second step is the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com