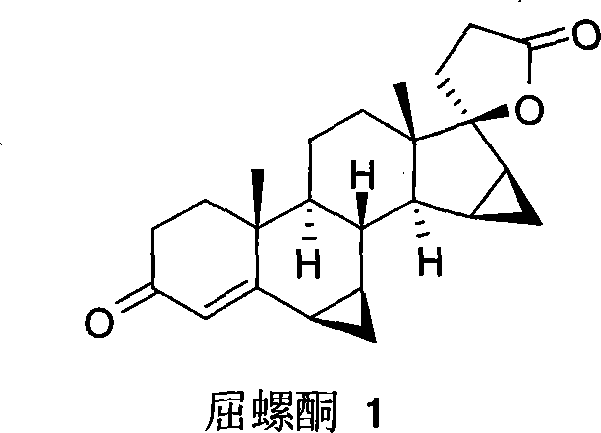
Novel method for stereo-selective chemosynthesis of drospirenone
A technology of stereoselectivity and drospirenone, which is applied in the production of organic chemistry, steroids, bulk chemicals, etc., can solve the problems of no innovative synthetic method reported, insufficient stereoselectivity, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
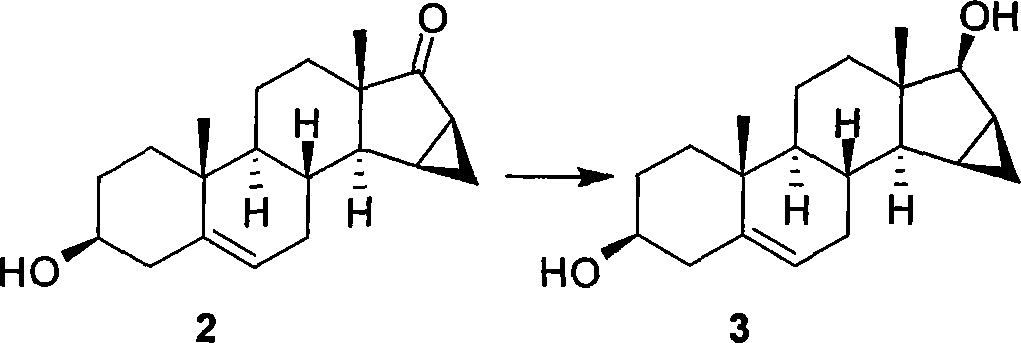
[0084] Example 1: Preparation of dihydroxy compound 3 (3β, 17β-dihydroxy-15β, 16β-methylene-androstane-5-ene)
[0085]
[0086] Compound 2 (40.0g, 0.1333mol) was dissolved in methanol (700mL) to form a suspension and cooled to 0°C, NaBH was added in batches 4 (5.544g, 0.1467mol) was reacted for 1 hour, and the suspension was clear at this time, and the raw materials were detected by TLC to disappear, so the reaction was stopped. saturated NH 4 Cl aqueous solution (80 mL) was used to quench the reaction, most of the solvent was spin-dried under reduced pressure, the reaction system was poured into a large amount of ice water, the solid was filtered and dried to obtain compound 3 (39.861 g) as a white solid with a reaction yield of 99%.
[0087] Compound 3: Mp 209-211°C; [α] 26 D -55.4 (c 0.37, CHCl 3 );
[0088] 1 H NMR (Py-d 5 , 300MHz) δ5.48(d, J=5.4Hz, 1H), 4.27(d, J=4.5Hz, 1H), 3.87(m, 1H), 1.07(s, 3H), 1.06(s, 3H), 0.30(dd, J=7.2, 13.2Hz, 1H);
[0089] 13 C NM...
Embodiment 2
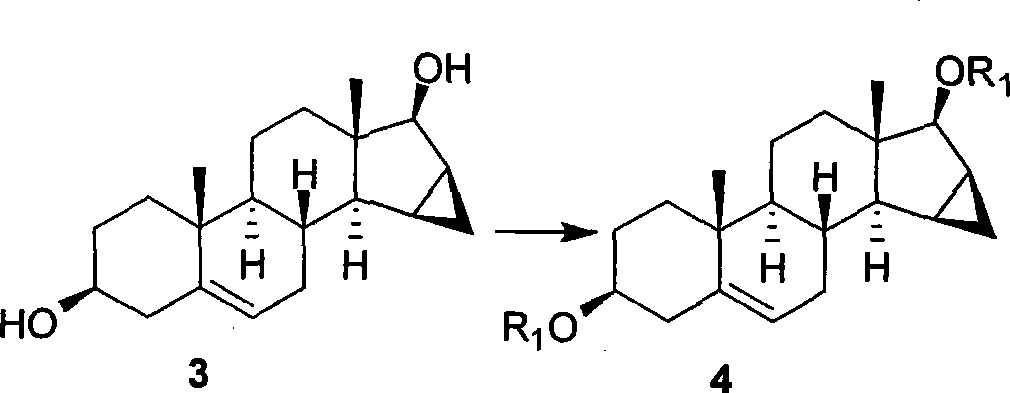
[0092] Example 2: Preparation of Compound 4 (3β, 17β-bis-TBSyloxy-15β, 16β-methylene-androstane-5-ene)
[0093]
[0094]Compound 3 (13.699g, 0.0454mol) and imidazole (15.40g, 0.227mol) were dissolved in anhydrous DMF (200mL) solvent, and tert-butyldimethylsilyl chloride (20.41g, 0.136mol) was added at room temperature. After stirring and reacting for 5 hours, it was detected by TLC that the reaction was found to be complete, and the reaction was stopped. The reaction system was poured into a large amount of ice water, and a large amount of white solid was precipitated at this time. The solid was collected by filtration and dried to obtain compound 4 (23.812 g) as a white solid with a yield of 99%.
[0095] Compound 4: mp 164-165 °C; [α] 26 D -18.1 (c 1.58, CHCl 3 );
[0096] 1 H NMR (CDCl 3 , 300MHz) δ5.36(d, J=4.8Hz, 1H), 3.95(d, J=4.5Hz, 1H), 3.48(m, 1H), 1.00(s, 3H), 0.91(s, 9H), 0.89(s, 9H), 0.76(s, 3H), 0.17(m, 1H), 0.07(d, J=9.9Hz, 6H), 0.06(s, 6H);
[0097] ...
Embodiment 3
[0101] Example 3: Preparation of Compound 5 (3β, 17β-bisTBSyloxy-15β, 16β-methylene-androstane-5-en-7-one)
[0102]
[0103] Compound 4 (3.642g, 6.872mmol), N-hydroxysuccinimide (NOS, 2.371g, 0.0206mol) were dissolved in acetone (40mL), and sodium dichromate (2.662g, 8.933mmol) was added at room temperature After the reaction was heated to 40°C and stirred for 36 hours, the reaction was stopped, and the reaction was quenched with saturated aqueous sodium sulfite (60 mL), and the reaction system was poured into a large amount of ice water. At this time, a large amount of white solid was precipitated. The solid was filtered, washed with water, dried, and the silica gel H column layer Analysis (petroleum ether: ethyl acetate = 50:1) separated and obtained white solid compound 5 (1.873g), the yield was 50%; recovered raw material 4 (1.743g, 47.9%).
[0104] Compound 5: mp 205-207°C; [α] 27 D -50.3 (c 0.52, CHCl 3 );
[0105] 1 H NMR (CDCl 3 , 300MHz) δ5.71(s, 1H), 3.95(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com